- Research
- Open access
- Published:
Surface-engineered bio-manufactured gas vesicles for multimodal imaging of glioma
Journal of Nanobiotechnology volume 23, Article number: 116 (2025)
Abstract
Integrated imaging techniques offer enhanced medical insights into the central nervous system by combining different modalities. In glioma diagnosis, the challenge often lies in delivering contrast agents effectively across the blood-brain barrier. We present an integrated multimodal imaging biohybrid, GV@pCY5, which enables blood-brain barrier penetrating as well as fluorescence and ultrasound (FL/US) imaging capabilities. This biohybrid is created by decorating a far-red-fluorescent cyanine dye (CY5) onto polyethyleneimine (PEI)-coated gas vesicles (GV). The layer-by-layer assembly improves the stability and performance of GV@pCY5 under ultrasound, thanks to the hydration shell variation induced by PEI. Given to the blood-brain barrier penetrating ability, GV@pCY5 demonstrates increase both in fluorescence and ultrasound imaging performance compared to single-component systems, proving effective for glioma diagnosis in vivo. This study underscores the potential of the FL/US platform for dual ratiometric imaging of various cerebral conditions.
Introduction
Glioma is the most common cancer of the central nervous system, comprising 80% of malignant primary brain tumors, with an incidence of 7 per 100,000 people [1, 2]. The survival rate for glioma patients is notably low, primarily due to the challenges in early and precise diagnosis of brain tumors [3]. This diagnosis heavily depends on advanced imaging techniques, which face two main challenges: (a) Difficulty in delivering contrast agents effectively to the glioma site to produce clear diagnostic signals. (b) The limitations of single imaging techniques, which often provide insufficient information for accurate diagnosis. As a result, there is an urgent need to develop new imaging tools that offer high-resolution capabilities for deep brain tumors.
Fluorescence (FL) and ultrasound (US) imaging strategies offer notable advantages in penetrating deep tissues, demonstrating significant potential for tumor diagnosis [4, 5]. Combining these two imaging modalities has proven effective in capturing and analyzing deep tissue information. For example, FL/photoacoustic dual-modal imaging has successfully realized non-invasive visualization of deepth atherosclerotic plaques and acute liver injury [6, 7]. Generally, far-red dyes are advantageous in FL imaging, due to their low background fluorescence, high signal clarity, and ability to penetrate deeper into tissue, such as cyanine dyes [8]. However, the diagnostic accuracy of FL/US imaging for orthotopic gliomas is still challenged by the limited accumulation of contrast agents in the tumor due to the tight junctions of the blood-brain barrier [9, 10]. While drug delivery systems, such as all-in-one nanoplatforms, show promise for improving delivery efficiency and imaging performance [11, 12], their complex components and unpredictable metabolic pathways pose challenges for potential clinical applications [13, 14].
Recent advancements in US imaging leverage micro- or nanometer-sized bubbles filled with gas, which significantly enhance imaging performance [15, 16]. Gas vesicles (GV), a unique class of gas-filled protein nanobubbles derived from microorganisms, offer notable ultrasound contrast under ultrasonic impact [17, 18]. GVs are remarkable biological structures with stability and flexibility, and safety, offer a potential alternative for potential clinical applications. Besides, GVs possess abundant active groups, such as -NH2 and -COOH, allowing functionalization with various molecules. For instance, hyaluronic acid can be grafted on GVs, permitting enhanced ultrasound contrast imaging and targeted drug delivery to the glioma, through binding to CD44 receptor on CD44-overexpressing glioma cells [19]. Among the various macromolecules, polyethyleneimine (PEI) is particularly promising for improving brain targeting due to its rapid cellular uptake and biocompatibility [20]. Its cationic charges facilitate stable interactions between proteins and lipophilic molecules [21, 22]. In this context, our work introduces a novel nanoplatform designed for FL/US dual-modal glioma imaging. This platform involves decorating a cyanine dye (CY5) onto PEI-coated GVs (GV@pCY5) using a layer-by-layer (LBL) assembly approach (Fig. 1). GV@pCY5 benefits from the structural stability imparted by PEI, which enhances its resistance to ultrasonic peening and improves ultrasound contrast performance. Additionally, the combination of biochemical and nanostructural features of GV@pCY5 facilitates more efficient internalization by GL261 cells compared to free CY5. The dual advantages of effective imaging and enhanced tumor cell penetration make GV@pCY5 a promising tool for reliable diagnosis of both subcutaneous and orthotopic gliomas with high sensitivity.
Materials and methods
Materials
PEI (M.W. 70 kD) was obtained from Macklin. Agarose and CY5 were purchased from Sigma-Aladdin. Dulbecco’s Modified Eagle Medium (DMEM) with high glucose, 0.25% trypsin, fetal bovine serum (FBS), and 100× penicillin-streptomycin (PS) were sourced from Gibco. PI and Hoechst 33,342 were purchased from Beyotime. Microcystis aeruginosa (M. aeruginosa) FACHB-2329 was obtained from the Wuhan Freshwater Algae Repository and cultured in BG11 medium at 25℃ in a light-controlled incubator. All chemicals were used without further purification.
Synthesis of GV@pCY5
GV was extracted from M. aeruginosa according to the literature [23]. Briefly, M. aeruginosa were transferred to a separating funnel after a 15-day cultivation at 25oC in an illuminating incubator. The medium was removed when M. aeruginosa float up to the surface, and then 30% H2O2 was mixed with the residual culture medium at the ratio of 1:10. The mixture was centrifugated at 400 ×g after they were incubated for 4 h, and then the white floats were collected. PBS was mixed with white float at a ratio of 1:1, and pure GVs were obtained by centrifugation at 400 ×g for 10 min. GVs were stored at 4℃ before use.
To obtain GV@PEI, diluted PEI concentrations, including 1.25, 2.50, 3.75, 5.00, and 7.50 µg/mL, were added to dispersions of GV (OD500 = 2.0) at room temperature. Then the dispersions were shaken for 2 h, and GV@PEI was obtained by collecting white floats. For synthesizing GV@pCY5, 15 µL of CY5 (2.5 mg/mL) was added into the dispersion of GV@PEI. Then GV@pCY5 was obtained after the mixture was shaken for 16 h.
Characterization GV@pCY5
The morphology of GV and GV@pCY5 was observed under TEM (HT7700). 10 µL of GV and GV@pCY5 (OD500 = 2.0) was dipped on a 200-mesh copper mesh and stained with 2% phosphotungstic acid, and then observed under 120 kV. The average size and z-potential of GV, GV@PEI, and GV@pCY5 were measured by Malvern zetasizer nano ZS90. FITR spectra of GV and GV@pCY5 were recorded with a Shimadzu FITR-8400 S from 4000 cmto 1 to 500 cm-1. UV-vis spectra of CY5 and GV@pCY5 were detected by a UV-vis spectroscopy (Cary 60), and the spectra were recorded from 450 to 850 nm. The FL spectra of CY5 and GV@pCY5 were recorded with a spectrofluorometer equipped with 450 W xenon lamp (RF6000).
Gel models of CY5, GV, GV@PEI, and GV@pCY5
To create phantoms for in vitro ultrasound imaging, gel models of CY5, GV, GV@PEI, and GV@pCY5 were established according to the literature [24]. Briefly, 1% agarose was filled into a plastic box, and wells were formed by poking with pipette tips post solidification. Next, 300 µL of samples in different concentration were loaded into 1% agarose and pipetted into the wells before solidification. Then the samples were sealed with 1% agarose to prevent their leakage, and stored at 4oC before analysis.
FL and US imaging of gel models
FL images of gel models of GV, CY5, and GV@pCY5 were obtained through a PerkinElmer IVIS spectrum. The gel models were put into the sample warehouse, and the FL images were recorded by Living image 4.4 at a FL channel of CY5.5. FL intensity of samples were analyzed by Living image 4.4. Ultrasound images of gel models were obtained by using a Mindray DP-50Vet portable system equipped with a linear transducer (75L38EB, 5.0–10.0 MHz). Different output power was applied to acquire US images of gel models containing GV, GV@PEI, and GV@pCY5 at a mechanical index of 0.2, including 25%, 34%, 55%, 67%, 89%, 100%, with a 75L38EB transducer at 7.5 MHz. Each image captured a circular cross-section of a well with a 4 mm diameter and centre positioned at a depth of 8 mm. Both gray and colorize images were recorded by the portable system, and then IntDen of the US images was calculated by ImageJ V1.8.0.
Cell culture and cytotoxicity
Raw264.7 and GL261 cells were cultured in complete high glucose DMEM medium supplied with 10% FBS and 1% PS at 37oC in a CO2 incubator. The cytotoxicity of GV, GV@PEI, and GV@pCY5 was evaluated by using a CCK-8 kit. In general, 1 × 104 of Raw264.7 or GL261 cells were added into a 96-well plate and cultured for 12 h, respectively, and then GV, GV@PEI, and GV@pCY5 at 2.0 OD500 were added into the 96-well plate with three parallels, and incubated for another 24 h. PBS was used as a control, and DMEM medium without cells were used as blank groups. 10% CCK8 in complete DMEM was added into the plate followed by washing with PBS for three times. The OD450 of the 96-well plate was measured by a microplate reader post a 45-min incubation at 37oC, and then the cell viability was calculated.
Live dead staining
1 × 105 of Raw264.7 cells were added into the dishes with a Gram-free coverslip, and cultured for 12 h before use. 400 µL of GV and GV@pCY5 (2.0 OD500) was added into the dishes with three parallels, and incubated for 4 h. Then 100 µL of PI (10 µg/mL) and Hoechst 33,342 (10 µg/mL) was added into the dishes followed by washing with PBS for three times. The coverslips were observed under FL microscopy (live cells, emission: 405 nm; dead cells, emission: 562 nm) after a 30-min incubation.
Hemolysis analysis
The hemolysis of GV, CY5, and GV@pCY5 was measured by adding them into the red blood cells. Red blood cells were collected from the orbital blood by centrifugation at 8000 ×g at 4oC for 5 min, and then washed with PBS for three times. The blood cells were divided into five groups with three parallels. GV (2.0 OD500), CY5 (75 µg), and GV@pCY5 (2.0 OD500) were used to disperse blood cells. Water and PBS were used as positive and negative controls. The treated red blood cells were incubated at 37oC for 1 h, and then centrifugated at 8000 ×g at 4oC for 5 min. The absorbance of the supernatant was measured at 670 nm, and hemolysis rate was calculated.
Cell uptake
Cellular uptake of GV@pCY5 were investigated by confocal microscopy and flow cytometry. 1 × 105 of GL261 were added into the 24-well plate and cultured for 12 h at 37oC. 100 µL of CY5 (7.5 µg/mL) and GV@pCY5 (CY5, 7.5 µg/mL) was added into the 900 µL of medium with three parallels, and cultured for 0.5 and 1.0 h, respectively. Cells were harvested and washed with PBS for three times post incubation. Finally, the cells were analyzed by flow cytometry, and 10,000 cells were gated. CY5+ cells and MFI value were calculated.
For fluorescence imaging, 1 × 105 of GL261 or Raw264.7 cells were added into the 3.5-cm dishes with glass bottom, and then cultured for 12 h at 37oC. 100 µL of CY5 (7.5 µg/mL) and GV@pCY5 (CY5: 7.5 µg/m) was added into the medium, and cultured for 0.5 h. 100 µL of Hoechst 33,342 (10 µg/mL) was added into the dishes and incubated for 10 min after the cells were washed with PBS. Cells were observed and recorded via a confocal microscopy (Leica TCS SP8). FL Intden of the treated cells was measured with ImageJ V1.8.0.
Besides, 1 × 105 of L929 or CT26 cells were incubated in 24-well plates, and then 100 µL of CY5 (7.5 µg/mL) and GV@pCY5 (CY5, 7.5 µg/mL) was added into the medium with three parallels. Cells were harvested at prearranged time points, and then detected with flow cytometry, in which 10,000 cells were gated for further analysis.
10 blood-brain barrier penetration ability of GV@pCY5
Brain-derived endothelial cells (bEnd.3 cells) were cultured in complete DMEM medium supplied with 10% FBS and 1% PS at 37oC in a CO2 incubator. The blood-brain barrier was established by seeding bEnd.3 cells into the uplayer of the transwell plate (0.4 μm pore size, 1 × 108 cm–2 pore density, 8.4 mm diameter) at a concentration of 1 × 105 cells per well. Media were changed every other day and cells were cultured for 9–11 days. Before the penetration studies, the endothelial paracellular barrier function was evaluated by measuring the peimeability of FITC-dextran. The inserts were transferred into another 24-well plate cultivated with 2 × 105 of GL261 cells, and then the transport abilities of CY5 and GV@pCY5 were studied by adding 200 µL of DMEM medium containg CY5 and GV@pCY5 (CY5: 0.75 µg/mL) to the uplayer of the insert. At 1, 2, and 4 h post incubation, the basal media and cells were collected for further analysis. The basal media were measured by using a fluorescence spectrophotometer and DLS. The basal cells were anlyzed by flow cytometry.
11 animals and tumor models
Balb/c mice (female, 4–6 weeks old) and C57BL/6J mice (female, 4–6 weeks old) were provided by Hunan Silaike jingda Experiment Animal Co. Ltd. All the animal experiments were approved and conducted in accordance with the guidelines and recommendations from the ethical committee of the Hainan University (Approve number: HNUAUCC-2023-00172). Mice were raised at 26oC with a 13 h:11 h light-dark photoperiod 16/8 h for 7 days before experiment. To establish subcutaneous tumor models, 100 µL of GL261 cells (1 × 107 cells mL− 1) were subcutaneously injected into the right flank of Balb/c mice, and then permitted to grow for about 7 days. For building orthotopic tumor models, 5 µL of GL261 cells (1 × 105 cells mL− 1) were intracerebrally injected into the anesthetized mice, and permitted to grow for about 10 days.
12 FL and US imaging in vivo
In vivo FL images of both subcutaneous and orthotopic Gl261 tumor-bearing mice were acquired using a PerkinElmer IVIS spectrum. Typically, 100 µL of PBS, CY5, and GV@pCY5 dispersion (GV: 2.0 OD500, CY5: 75 µg mL− 1, n = 3) were injected intravenously into the anesthetized mice with 1.5% pentobarbital (100 µL). FL imaging images were obtained at different time intervals post injection. The FL intensity of the tumor site were analyzed by Living imaging 4.4. Main organs and tumors were collected from the sacrificed mice at 24 h post injection. Similarly, the FL imaging images of tissues were recorded and analyzed by the software.
In vivo US images of both subcutaneous and orthotopic Gl261 tumor-bearing mice were obtained using a Mindray DP-50Vet portable system equipped with a convex array transducer (65C15EA, 5.0-8.5 MHz). Typically, 100 µL of PBS, GV, and GV@pCY5 dispersion (GV: 2.0 OD500, CY5: 75 µg mL− 1, n = 3) were injected via tail vein into the anesthetized mice with 1.5% pentobarbital (100 µL). US imaging images were obtained at 8 h post injection at an output power of 55% at 7.5 MHz (MI = 0.2), and IntDen of the tumor site were analyzed ImageJ.
13 histology and biochemicals analysis
The obtained tissues, including livers, spleens, kidneys, and brains, were fixed with paraformaldehyde, embedded with paraffin, and prepared into 10-µm sections. The sections were stained with HE, and observed under digital pathology slide scanner (Aperio LV1 IVD). The whole blood stored in ethylenediaminetetraacetic acid anticoagulant tube was analyzed by Mindary automatic blood cell analyzer (BC-760 CS). Besides, serum analysis was performed on automatic biochemical analyzer (BS-600 M), including alanine transaminase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and uric acid (UA).
14 staining of brain in orthotopic glioma models
The brains of orthotopic glioma tumor model, which were treated with PBS, CY5, and GV@pCY5, were fixed with paraformaldehyde and embedded with paraffin, and then sectioned into 10-µm sections. The sections were stained with DAPI (10 µg/mL) for 10 min after dewaxing process, and then observed under FL microscopy. Images were recorded by Nikon ECLIPSE Si system upon excitation at 488 nm and 536 nm.
15 data analysis
All data were expressed as mean ± standard deviation (SD), and at least three samples were included in each statistical analysis. Student’s T-test was used for comparison between two groups, and one-way analysis of variance with Tukey’s post-test was used for comparisons between more than two groups. Statistical analysis was performed by using the Prism 9. Differences were significant when the p-value was ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p < 0.001, or ∗∗∗∗p < 0.0001.
Results and discussion
Characterization of GV@pCY5
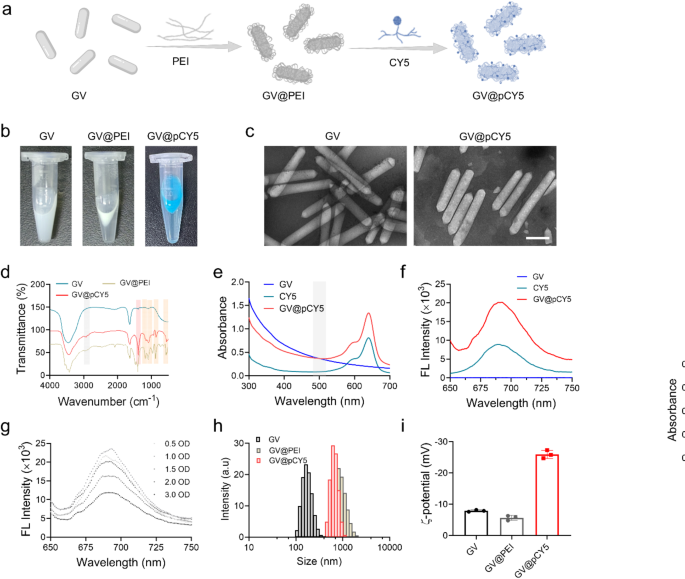
GVs are rod-shaped, gas-filled protein nanostructures that have been used in ultrasound-triggered biomedical applications [25]. CY5, a far-red fluorescent dye, is an effective tracer for peptides and proteins in vivo due to its high tissue penetration capabilities for bioimaging [26]. Leveraging the unique properties of GVs and CY5, we developed a dual-modal imaging probe, GV@pCY5, through layer-by-layer (LBL) assembly (Fig. 2a). In this process, PEI served as both a linker and a protective agent. GVs extracted from M. aeruginosa were initially in the form of a milky white suspension in PBS. After PEI grafting via electrostatic adsorption, the suspension quickly floated, and turned blue following CY5 assembly (Fig. 2b), indicating the successful synthesis of GV@pCY5. The GVs and GV@pCY5 were typically rod-shaped, measuring approximately 80 nm in width and 300–800 nm in length. Compared to the morphology of GV, GV@pCY5, due to the assembly of PEI and CY5, presented multiple surface dots (Fig. 2c), yet retained a similar shape to GV, indicated that the synthesis of GV@pCY5 minimally affects the original structure and morphology of GV [27].
Characterization of GV@pCY5. a Schematic illustration of self-targeting GV@pCY5 for diagnosis of glioma. b Photograph of GV, GV@PEI, and GV@pCY5 in PBS. c Representative transmission electron microscopy (TEM) images of GV and GV@pCY5. Scale bar: 200 nm. d Fourier transform infrared (FTIR) spectra of GV, GV@PEI, and GV@pCY5. e, f Ultraviolet-visible (UV-vis) (e) and FL (f) spectrum of GV, CY5 and GV@pCY5. Excitation: 630 nm. g FL spectrum of GV@pCY5 at different concentrations. Excitation: 630 nm. h, i Size distribution (h) and z-potential (i) of GV, GV@PEI and GV@pCY5
Electrostatic interactions between the negatively charged GV and positively charged PEI during the preparation of GV@PEI can impact the molecular exchanges on the protein shell of the GVs, potentially altering their stability. To explore the effect of PEI density on GV stability, we assessed the size distribution and US imaging performance of GV@PEI synthesized with varying PEI concentrations. The PEI shell, which scatters light upon laser irradiation, resulted in a larger hydrated size for GV@PEI compared to bare GVs. The smallest average hydrated size and greatest stability were observed at a PEI concentration of 3.75 µg/mL (Figure S1 and Figure S2). Additionally, GV@PEI synthesized with 3.75 µg/mL PEI demonstrated the strongest US imaging contrast (Figure S3 and Figure S4). We supposed that PEI strengthened US imaging by stabilizing the inherent structure of GV by interacting with gas vesicle proteins. Nevertheless, the biocompatibility of PEI is still challenging the in vivo applications although it was considered as a favorable macromolecule for biomedical applications [28,29,30]. We further evaluated the cytotoxicity of GV@PEI (OD500 = 2.0) synthesized from gradient PEI, and found that GV@PEI hardly affected the cell viability of Raw264.7 and GL261 cells even at a high PEI concentration up to 7.50 µg/mL (Figure S5). Overall, PEI at a concentration of 3.75 µg/mL performed not only as a linker between GV and CY5, but also improved the US imaging capabilities while maintaining low cytotoxicity.
By virtue of the high affinity of PEI with lipid-soluble dyes [31], CY5 was linked onto GV@PEI to generate GV@pCY5. To confirm the linkage between CY5 and GV, we recorded the FTIR spectra of GV, GV@PEI, and GV@pCY5. Peaks at 2935 and 2832 cm-1 appeared in the FTIR spectra of GV@PEI and GV@pCY5 (Fig. 2d), contributing to the stretching vibration of -CH2 in PEI [32]. Besides, peak at 1190 cm-1 in the spectrum of GV@PEI and GV@pCY5 belonged to the C-N stretching vibration peaks of PEI and CY5. Furthermore, peaks at 1468, 950, 721, 636 cm-1 indicated bending vibration of -NH disappeared in the spectrum of GV@pCY5, and peaks at 1350, 1077, 860, and 547 cm-1 indicated the existence of -SO2-, S = O, S-O, and S-C, ascribing to the characteristic structure of CY5 [33]. The above results suggested that CY5 was linked on GV via physical interaction without forming new chemical bond. We then verified the existence of CY5 on GV by detecting the UV-vis and FL spectrum of CY5 and GV@pCY5. A representative absorbing peak of CY5 can be observed at 630 nm on the UV-vis spectra of GV@pCY5 (Fig. 2e), and the emission peak at 690 nm was detected on the FL spectra of GV@pCY5 (Fig. 2f). Besides, GV@pCY5 (OD500 = 2.0) showed a 2.0-fold FL enhancement compared to free CY5, implying that the nanostructured GV could improve the FL intensity of CY5 (Fig. 2f and g). Although GV showed a similar morphology to GV@pCY5 in TEM images, their hydrated diameters were 182.8 ± 9.2 and 808.4 ± 44.9 nm, respectively (Fig. 2h). The larger size of GV@pCY5 could be explained by the formation of PEI hydration layer on GV. The z-potential of hybrids decreased largely from − 7.50 ± 0.30 to -25.9 ± 1.13 mV following decoration with PEI and CY5 (Fig. 2i). Taken together, the stabilized dual-modal FL/US imaging probe was successfully synthesized by LBL assembly, which possessed higher FL and US imaging ability in comparison to free CY5 and bared GV.
FL and US imaging performance of GV@pCY5
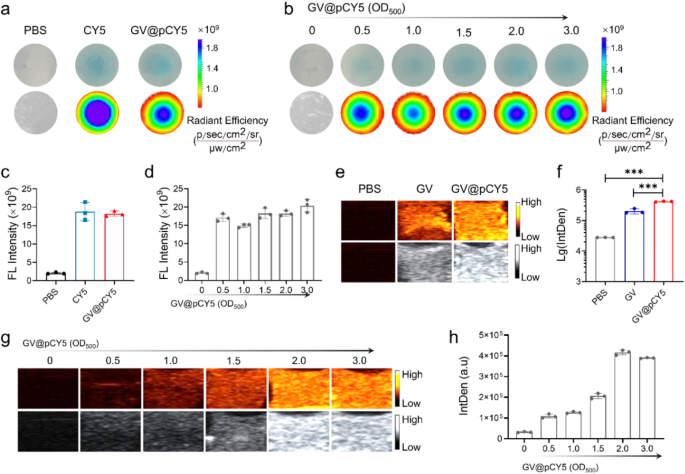
To assess the in vitro FL/US imaging capacity of the dual-modal imaging probe, we recorded the FL and US images of GV@pCY5 by living imaging devices. PBS, CY5, and GV@pCY5 were sealed in gels made from agarose to simulate tumors before FL and US imaging according to the literature [24]. When sealed in the in vitro gel models, GV@pCY5 possessed a more even FL imaging capacity than free CY5 (Fig. 3a) together with an equal FL intensity (Fig. 3c), illustrating that GV@pCY5 could pose a prospect in FL imaging. The imaging ability of FL imaging probes would be influenced by their concentration [34], therefore, we evaluated the corresponding impact of the concentration on FL imaging of GV@pCY5. In particular, the FL imaging ability of GV@pCY5 showed a dose-independent behavior, as they showed similar FL images (Fig. 3b) and equivalent FL intensity (Fig. 3d).
FL/US imaging capacity in vitro. a, c FL imaging (a) and FL intensity (c) of PBS, CY5, and GV@pCY5 sealed in agarose gels. b, d FL imaging (b) and FL intensity (d) of GV@pCY5 sealed in agarose gels in 0, 0.5, 1.0, 1.5, 2.0, and 3.0 OD500. e, f US imaging (e) and US IntDen (b) of PBS, CY5, and GV@pCY5 sealed in agarose gels. g, h US imaging (g) and US IntDen (h) of GV@pCY5 sealed in agarose gels in 0, 0.5, 1.0, 1.5, 2.0, and 3.0 OD500. n = 3, ***, p ≤ 0.001
We investigated the suitable output power of Mindray portable ultrasound machines by applying series of output power on agar models [35]. The IntDen of both GV and GV@pCY5 increased along with the enlarged output power before a 55%-output power, and then decreased gradually (Figure S6). Consequently, an output power of 55% was selected to assess the US contrast efficiency both in vitro and vivo. GV and GV@pCY5 showed brightest US signals (Fig. 3g and Figure S7) and highest IntDen (Fig. 3h and Figure S8) at a concentration of 2.0 OD500. Consequently, we applied GV@pCY5 at a concentration of 2.0 OD500 to conduct the cellular and in vivo imaging experiment with an output power of 55%. Remarkably, GV@pCY5 owned an improved US imaging ability compared with bared GV (Fig. 3e and f), due to the invigorating effect of PEI on protein skeleton, which was further confirmed by the focused ultrasonic treatment. Upon US treatment, GV@pCY5 prepared from diluted PEI kept their milk-white color; whereas GVs turned transparent (Figure S9), contributing to the damage of the vesicle structure, which indicated that GV@pCY5 possessed a better anti-ultrasonic stripping property compared with bared GV. Collectively, GV@pCY5 showed enhanced FL/US imaging capacity with higher homogeneity compared with bared GV and free CY5 in imaging devices, indicating the potential application in enhanced dual-modal imaging.
Cytotoxicity, cell uptake and penetrability of GV@pCY5
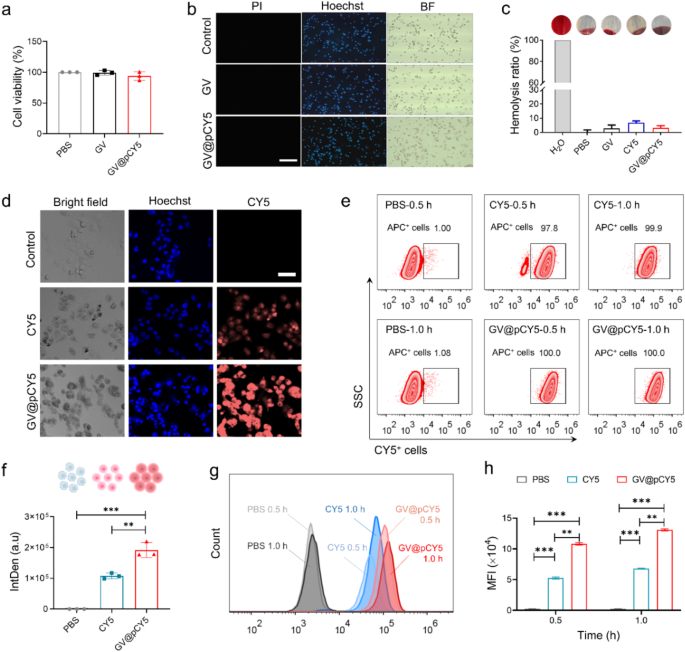
The imaging capacity of probes was related to their cell viability and efficient cellular uptake [36, 37], thus we measured the cytotoxicity, cellular uptake, as well as hemolysis properties of GV@pCY5 to evaluated its applicability. GV@pCY5 at a concentration of 2 OD500 showed no obvious impact on cell viability of Raw267.4 and GL261 cells post a 24-h-treatment (Figure S10 and Fig. 4a), indicating its biocompatibility at the cellular level. Live-dead staining of Raw267.4 cells treated by GV@pCY5 showed the same result. We treated 1 × 105 of Raw267.4 and GL261 cells with GV and GV@pCY5 at a concentration of 2.0 OD500 for 24 h, and then stained the cells with mixture of pyridine iodide (PI) and Hoechst 33,342 (Hoechst). Under FL microscopy, living and dead cells appeared as blue and red color. The Raw267.4 cells still alive after they were treated by GV and GV@pCY5 (Fig. 4b), demonstrating the ultralow cytotoxicity of GV@pCY5 toward Raw267.4 and GL261 cells. To assess the practical possibility, we detected the hemolytic rate of GV, CY5, and GV@pCY5 by incubating them with red blood cells. PBS and water were used as negative and positive controls. Both GV and GV@pCY5 hardly triggered hemolysis as their hemolytic rate was less than 5%; CY5 would cause an approximately 7% hemolytic rate (Fig. 4c). The results proved that GV fixed CY5 showed a decreased hemolytic rate compared with free CY5, illustrating a higher biocompatibility of GV@pCY5 [38]. Consequently, GV@pCY5 were biocompatible to Raw264.7 and GL261 and red blood cells when their concentration was 2.0 OD500, which would benefit the further in vivo applications.
Insufficient cellular uptake by target cells has hindered the clinical application of nanostructured imaging probes [39]. To address this, we evaluated the cellular uptake of CY5 and GV@pCY5 in GL261 and Raw264.7 cells. Compared to GL261 cells treated with free CY5, those treated with GV@pCY5 exhibited significantly higher fluorescence intensity (Fig. 4d). Specifically, a substantial increase in fluorescence was observed in GL261 cells treated with GV@pCY5 compared to free CY5 (Fig. 4f), indicating a preference of GL261 cells for nano-sized biohybrids over free dyes. In contrast, Raw264.7 macrophages, L929 fibroblasts, and CT26 colorectal cancer cells showed similar cellular uptake efficacy for both GV@pCY5 and free CY5, as evidenced by comparable average fluorescence intensities in these cells (Figure S11, S12, and S13). Additionally, flow cytometry analysis revealed that GVs facilitate the entry of CY5, with CY5+ cells treated with GV@pCY5 demonstrating 100% cellular uptake within 0.5 h (Fig. 4e). Moreover, the MFI of GL261 cells treated with GV@pCY5 was 2.2-fold higher than that of cells treated with free CY5 (Fig. 4g and h), further confirming the enhanced cellular uptake by GL261 cells. Overall, GV enhances the cellular uptake of CY5 and shows good cell and blood compatibility, underscoring its potential significance for bioimaging [40].
Biocompatibility and cellular uptake and penetrability of GV@pCY5. a Cell viability of GL261 cells treated by PBS, GV, and GV@pCY5 for 24 h. b Live/dead staining of Raw264.7 cells treated by PBS, GV, and GV@pCY5. Scale bar: 100 μm. c Hemolysis ratio of PBS, GV, CY5, and GV@pCY5. d, f Confocal images (d) and Intden (f) of GL261 cells treated with PBS, CY5, and GV@pCY5. Scale bar: 50 μm. e Flow cytometry assay of GL261 cells treated by PBS, CY5, and GV@pCY5 for 0.5 and 1.0 h. g, h Histogram (g) and mean FL intensity (MFI) (h) of CY5+ GL261 cells treated by PBS CY5, and GV@pCY5 for 0.5 and 1.0 h. n = 3, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
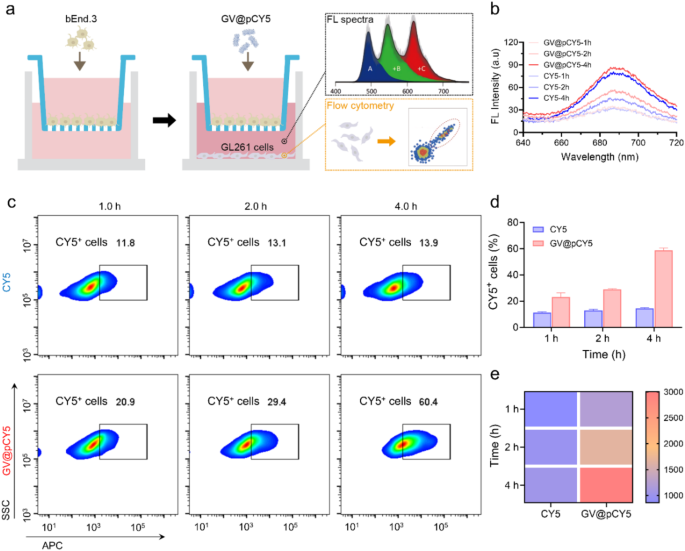
The blood-brain barrier limits the penetration of imaging probes, restricting their imaging efficacy [41]. To assess the ability of GV@pCY5 to cross this barrier, we established an in vitro model by seeding bEnd.3 cells in the inserts of a transwell plate and culturing them for 10 days. We then added GV@pCY5 to the inserts and collected the basal medium and cells at various intervals for fluorescence spectra, dynamic light scattering (DLS) analysis, and flow cytometry (Fig. 5a). Compared to free CY5, the basal medium treated with GV@pCY5 showed higher fluorescence at certain time points (Fig. 5b), indicating that GV@pCY5 has improved efficacy in penetrating the blood-brain barrier. Besides, the presence of PEI contributed to a higher particle count in the basal medium (Figure S14). Additionally, cellular uptake of GV@pCY5 in GL261 cells increased with incubation time, with approximately 60% of cells showing fluorescence after 4 h of incubation with GV@pCY5 (Fig. 5c and d), which is 40% higher than that observed with free CY5. The highest fluorescence was detected in GL261 cells treated with GV@pCY5 for 4 h (Fig. 5e). Overall, GV@pCY5 demonstrated superior penetration ability across the in vitro blood-brain barrier model compared to the free hydrophobic dye and showed increased uptake by glioma cells.
Penetration ability of GV@pCY5 across the in vitro blood-brain barrier. a Experimental Design. bEnd.3 cells were cultured in inserts within a transwell plate for 10 days until tight junctions were established. The inserts were then transferred into wells containing GL261 cells. Following incubation with PBS, CY5, and GV@pCY5, the basal medium and GL261 cells were collected at specified time points for further analysis. b Fluorescence spectra of the basal medium after incubation with CY5 and GV@pCY5 for 1, 2, and 4 h. c Flow cytometry analysis of GL261 cells following incubation with CY5 and GV@pCY5 for 1, 2, and 4 h. d, e The ratio of CY5+ cells (d) and fluorescence intensity (e) in GL261 cells after incubation with CY5 and GV@pCY5 for 1, 2, and 4 h
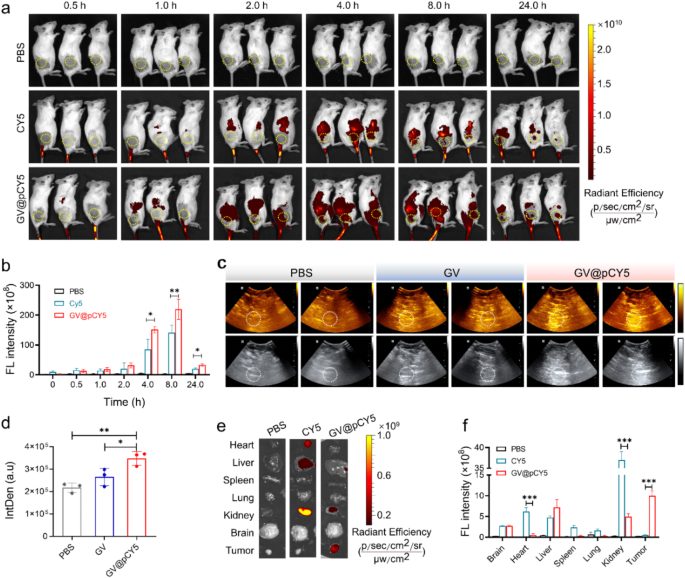
FL/US imaging in subcutaneous GL261 tumor model
We evaluated the stability of GV@pCY5 in FBS over 10 days by monitoring its average size and ζ-potential. GV@pCY5 maintained an average size of approximately 800 nm and a ζ-potential of about − 25.5 mV throughout the storage period (Figure S15), indicating good stability and suitability for in vivo applications. To preliminarily investigate the US/FL imaging performance of GV@pCY5, we treated the subcutaneous GL261 tumor-bearing mice by intravenous administration of PBS, CY5, and GV@pCY5 (100 µL, 2.0 OD500). We recorded the FL images by a small animal living imaging system of PerkinElmer in vivo imaging system (IVIS) Lumina III at 0.5, 1.0, 2.0, 4.0, 8.0, and 24.0 h post administration, and the average FL signal intensity in tumor site was measured by Living Image 4.0. The GL261 tumor-bearing mice showed strong fluorescence at 2.0 h post injection (Fig. 6a), contributing to the liver accumulation ability of probes [42]. The FL signal at tumor site reached highest level at 8.0 h and could still be observed even at 24.0 h post-injection (Fig. 6b). GV@pCY5 demonstrated higher fluorescence intensity at 4 h compared to free CY5, suggesting enhanced imaging efficiency. For ultrasound imaging, we used a Mindray DP-50 Expert portable ultrasound device at 8 h post-injection of PBS, GV, and GV@pCY5. GV@pCY5 produced brighter ultrasound signals at the tumor site, with greater average intensity compared to GV (Fig. 6c and d), indicating its superior ultrasound imaging capability. Generally, GV@pCY5 showed favorable capacity of dual-modal FL and US imaging performance, making it a valuable tool for tumor diagnosis.
We evaluated the distribution of GV@pCY5 by FL imaging of collected organs and tumors excised from the treated GL261 tumor-bearing mice at 24 h post injection. A bright FL signal was observed in livers and kidneys (Fig. 6e), indicating that the probes were cleared through hepatic and renal metabolism [43]. Remarkably, the average FL intensity at the tumor site of mice treated with GV@pCY5 was 3.4 times higher than that of the CY5 injected mice (Fig. 6f), illustrating excellent selective accumulation in tumors of GV@pCY5. These results demonstrated great potential of GV@pCY5 as an enhanced FL/US dual-modal imaging probe for tumor diagnosis.
GV@pCY5 enabled improved FL/US dual model imaging of subcutaneous tumors. a FL images of mice intravenously injected with PBS, CY5, and GV@pCY5 at different time intervals. b Fluorescence intensity of the tumor site at 0, 0,5, 1.0, 2.0, 4.0, 8.0, and 24.0 h post injection with PBS, CY5, and GV@pCY5. c, d Representative US images (c) and IntDen (d) of subcutaneous tumors of mice at 6 h post intravenous injection with PBS, GV, and GV@pCY5. e, f Fluorescence images (e) and fluorescence intensity (f) of main organs in mice sacrificed at 24 h post injection with PBS, CY5, and GV@pCY5. n = 3, *, p ≤ 0.05, **, p ≤ 0.01
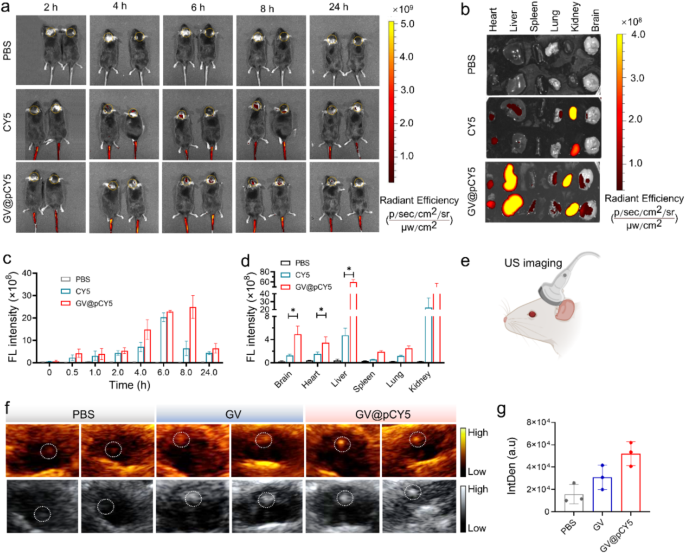
Enhanced FL/US imaging in orthotopic GL261 glioma model
The FL/US imaging ability has been proved both in vitro and in subcutaneous GL261 tumor-bearing models, we further evaluated the FL/US dual-mode imaging ability in orthotopic GL261 glioma models. Similarly, we recorded both FL and US images of orthotopic glioma models injected with GV@pCY5. Obvious FL signals in the tumor site appeared at 2 h post injection of CY5 and GV@pCY5, and showed highest FL intensity at 6 h post injection (Fig. 7a and c). Particularly, the tumor site of mice treated with GV@pCY5 possessed 3.0 times higher FL intensity compared with mice treated with CY5 at 8 h post injection (Fig. 7c), illustrating that GV@pCY5 displayed longer diagnosis window than free CY5 [44]. Besides, compared with free CY5, GV@pCY5 showed higher apparent FL signals at the tumor site of glioma models (Fig. 7b and d), indicating the FL imaging effectiveness of GV@pCY5. For US imaging, the mice were scanned with a Mindray DP-50 Expert portable ultrasound imaging device (Fig. 7e). The glioma displayed brightest US signals and clearest tumor boundary among mice treated by PBS, GV, and GV@pCY5 (Fig. 7f). Be different from FL imaging, US imaging of mice provided spatialization of glioma, which could strengthen fixed position diagnosis of intracranial tumors. At 6 h post intravenous administration, the IntDen of the tumor site of mice injected with GV@pCY5 was 2.2-fold higher than that of mice injected with GV (Fig. 7g), contributing to the high stability and US imaging capacity of GV@pCY5. The above results indicating that GV@pCY5 showed higher efficiency in glioma diagnosis.
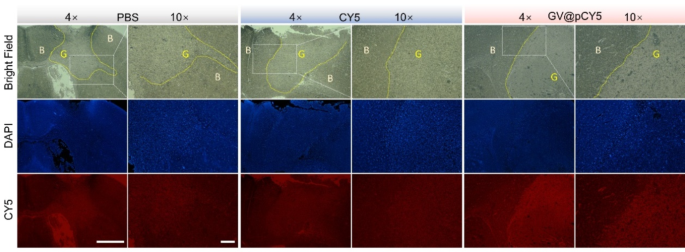
It was suspected that GV@pCY5 showed higher accuracy compared with free CY5, so we observed the fluorescence distribution in brain of glioma-bearing mice treated with PBS, CY5, and GV@pCY5. Higher red fluorescence was detected in tumors of glioma-bearing mice treated with GV@pCY5 in comparison to free CY5 and PBS (Fig. 8). Free CY5 hardly heightened the FL contrast between brain and glioma, whereas clear boundary appeared in tumors of glioma-bearing mice treated with GV@pCY5, ascribing to the penetrating ability of PEI-capped nano-sized hybrids. Moreover, high contrast between brain and glioma further verified the self-targeting ability of GV@pCY5. Taken together, the simultaneous dual-modal FL and MR imaging could provide more complementary information from each imaging modality for tumor diagnosis.
GV@pCY5 enabled improved FL/US dual model imaging of orthotopic glioma. a Fluorescence images of mice intravenously injected with PBS, CY5, and GV@pCY5 at different time intervals. b, d Fluorescence images (b) and fluorescence intensity (d) of main organs in mice sacrificed at 24 h post injection with PBS, CY5, and GV@pCY5. c Fluorescence intensity of the tumor site at 0, 0,5, 1.0, 2.0, 4.0, 8.0, and 24.0 h post injection with PBS, CY5, and GV@pCY5. e Diagram of US imaging of orthotopic tumors. f, g Representative US images (f) and IntDen (g) of orthotopic tumors of mice at 6 h post intravenous injection with PBS, GV, and GV@pCY5. n = 3, *, p ≤ 0.05, **, p ≤ 0.01
Biosafty of GV@pCY5
We further assessed the in vivo biosafety of GV@pCY5 by examining blood, serum, and tissue samples from mice that were treated with systemic administration of PBS, GV, and GV@pCY5. Analysis of blood samples from mice administered GV@pCY5 showed no significant changes in the complete blood count; the levels of white blood cells (WBC), red blood cells (RBC), platelets (PLT), and lymphocytes (Lymph) remained stable (Figure S16). HE staining analysis indicated that GV@pCY5 did not cause notable pathological damage to the liver, spleen, or kidneys compared to the control group (Figure S17a). Additionally, biochemical markers related to liver and kidney function, such as ALT, AST, BUN, and UA, were within normal ranges for mice treated with GV@pCY5, similar to those in the PBS group (Figure S17b-d). Elevated levels of these markers are typically associated with liver and kidney damage, further supporting the low toxicity and favorable in vivo safety profile of GV@pCY5 [45]. These findings suggest that GV@pCY5 is a safe and effective tool for in vivo glioma diagnosis.
Conclusion
In summary, we developed a biomimetic nanoplatform by using layer-by-layer (LBL) assembly of CY5 on GVs for US/FL multimodal glioma diagnosis. The resulting GV@pCY5 exhibited a uniform size and a ζ-potential of -29.5 mV. Thanks to the continuous polymer coating, GV@pCY5 demonstrated improved contrast enhancement in both US and FL imaging, with greater stability compared to bare GV. Additionally, GV@pCY5 showed enhanced cellular uptake, good biocompatibility, penetrating ability across blood-brain barrier and no significant hemolysis, making it suitable as a multimodal contrast agent. It enhanced the diagnosis of both subcutaneous and orthotopic glioma models, providing higher fluorescence intensity than free CY5 and increased IntDen in US imaging. These results offer valuable insights for developing biomimetic nanodrug carriers for accurate glioma diagnosis and potentially other brain-related diseases. Moreover, the ability of this platform to co-deliver drugs highlights its potential as a theragnostic tool for various conditions.
Data availability
No datasets were generated or analysed during the current study.
References
Gould J. Breaking down the epidemiology of brain cancer. Nature. 2018;561:S40–1.
Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15:422–42.
Yang Y, He MZ, Li T, Yang X. MRI combined with PET-CT of different tracers to improve the accuracy of glioma diagnosis: a systematic review and meta-analysis. Neurosurg Rev. 2019;42:185–95.
Gao S, Wang G, Qin Z, Wang X, Zhao G, Ma Q, Zhu L. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials. 2017;112:324–35.
Rabut C, Correia M, Finel V, Pezet S, Pernot M, Deffieux T, Tanter M. 4D functional ultrasound imaging of whole-brain activity in rodents. Nat Methods. 2019;16:994–7.
Yu Q, Duan Y, Liu N, Zhu Z, Sun Y, Yang H, Shi Y, Li X, Zhu W, Wang L, Wang Q. Fluorescence and photoacoustic (FL/PA) dual-modal probe: responsive to reactive oxygen species (ROS) for atherosclerotic plaque imaging. Biomaterials. 2025;313:122765.
Cai W, Sun J, Sun Y, Zhao X, Guo C, Dong J, Peng X, Zhang R. NIR-II FL/PA dual-modal imaging long-term tracking of human umbilical cord-derived mesenchymal stem cells labeled with melanin nanoparticles and visible HUMSC-based liver regeneration for acute liver failure. Biomater Sci. 2020;8:6592–602.
Li S, Sun Z, Deng G, Meng X, Li W, Ni D, Zhang J, Gong P, Cai L. Dual-modal imaging-guided highly efficient photothermal therapy using heptamethine cyanine-conjugated hyaluronic acid micelles. Biomater Sci. 2017;5:1122–9.
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–96.
Castagnola V, Deleye L, Podesta A, Jaho E, Loiacono F, Debellis D, Trevisani M, Ciobanu DZ, Armirotti A, Pisani F, et al. Interactions of Graphene Oxide and few-layer graphene with the blood-brain barrier. Nano Lett. 2023;23:2981–90.
Shang W, Zeng C, Du Y, Hui H, Liang X, Chi C, Wang K, Wang Z, Tian J. Core-shell gold nanorod@metal-organic framework nanoprobes for multimodality diagnosis of glioma. Adv Mater. 2017;29:1604381.
Zhu M, Sheng Z, Jia Y, Hu D, Liu X, Xia X, Liu C, Wang P, Wang X, Zheng H. Indocyanine green-holo-transferrin nanoassemblies for tumor-targeted dual-modal imaging and photothermal therapy of glioma. ACS Appl Mater Interfaces. 2017;9:39249–58.
Liu Y, Li K, Liu B, Feng SS. A strategy for precision engineering of nanoparticles of biodegradable copolymers for quantitative control of targeted drug delivery. Biomaterials. 2010;31:9145–55.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–24.
Wang R, Wang L, Chen Y, Xie Y, He M, Zhu Y, Xu L, Han Z, Chen D, Jin Q, et al. Biogenic gas vesicles for ultrasound imaging and targeted therapeutics. Curr Med Chem. 2022;29:1316–30.
Jo S, Sun IC, Ahn CH, Lee S, Kim K. Recent trend of ultrasound-mediated nanoparticle delivery for brain imaging and treatment. ACS Appl Mater Inter. 2023;15:120–37.
Schnell C. Gas vesicles enable ultrasound imaging. Nat Methods. 2018;15:159–159.
Mehta RI, Carpenter JS, Mehta RI, Haut MW, Wang P, Ranjan M, Najib U, D’Haese PF, Rezai AR. Ultrasound-mediated blood-brain barrier opening uncovers an intracerebral perivenous fluid network in persons with Alzheimer’s disease. Fluids Barriers CNS. 2023;20:46.
Lakshmanan A, Farhadi A, Nety SP, Lee-Gosselin A, Bourdeau RW, Maresca D, Shapiro MG. Molecular Engineering of Acoustic protein nanostructures. ACS Nano. 2016;10:7314–22.
Candela P, Gosselet F, Miller F, Buee-Scherrer V, Torpier G, Cecchelli R, Fenart L. Physiological pathway for low-density lipoproteins across the blood-brain barrier: transcytosis through brain capillary endothelial cells in vitro. Endothelium. 2008;15:254–64.
Duan QY, Zhu YX, Jia HR, Guo Y, Zhang X, Gu R, Li C, Wu FG. Platinum-coordinated dual-responsive nanogels for universal drug delivery and combination cancer therapy. Small. 2022;18:e2203260.
Hao F, Li Y, Zhu J, Sun J, Marshall B, Lee RJ, Teng L, Yang Z, Xie J. Polyethylenimine-based formulations for delivery of oligonucleotides. Curr Med Chem. 2019;26:2264–84.
Lakshmanan A, Lu GJ, Farhadi A, Nety SP, Kunth M, Lee-Gosselin A, Maresca D, Bourdeau RW, Yin M, Yan J, et al. Preparation of biogenic gas vesicle nanostructures for use as contrast agents for ultrasound and MRI. Nat Protoc. 2017;12:2050–80.
Bar-Zion A, Nourmahnad A, Mittelstein DR, Shivaei S, Yoo S, Buss MT, Hurt RC, Malounda D, Abedi MH, Lee-Gosselin A, et al. Acoustically triggered mechanotherapy using genetically encoded gas vesicles. Nat Nanotechnol. 2021;16:1403–U1147.
Ling B, Lee J, Maresca D, Lee-Gosselin A, Malounda D, Swift MB, Shapiro MG. Biomolecular ultrasound imaging of phagolysosomal function. ACS Nano. 2020;14:12210–21.
Pramanik A, Xu ZX, Shamsuddin SH, Khaled YS, Ingram N, Maisey T, Tomlinson D, Coletta PL, Jayne D, Hughes TA, et al. Affimer tagged cubosomes: targeting of carcinoembryonic antigen expressing colorectal cancer cells using in vitro and in vivo models. ACS Appl Mater Inter. 2022;14:11078–91.
Wang Y, Fu M, Yang Y, Zhang J, Zhang Z, Xiao J, Zhou Y, Yan F. Modification of PEG reduces the immunogenicity of biosynthetic gas vesicles. Front Bioeng Biotechnol. 2023;11:1128268.
Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–9.
Ramos J, Forcada J, Hidalgo-Alvarez R. Cationic polymer nanoparticles and nanogels: from synthesis to biotechnological applications. Chem Rev. 2014;114:367–428.
Dabbaghi M, Kazemi Oskuee R, Hashemi K, Afkhami Goli A. Evaluating polyethyleneimine/DNA nanoparticles-mediated damage to cellular organelles using endoplasmic reticulum stress profile. Artif Cells Nanomed Biotechnol. 2018;46:192–9.
Wong S, Tumari HH, Ngadi N, Mohamed NB, Hassan O, Mat R, Amin NAS. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J Clean Prod. 2019;206:394–406.
Bin Y, Liang Q, Luo H, Chen Y, Wang T. One-step synthesis of nitrogen-functionalized graphene aerogel for efficient removal of hexavalent chromium in water. Environ Sci Pollut Res Int. 2023;30:6746–57.
Wavhale RD, Dhobale KD, Rahane CS, Chate GP, Tawade BV, Patil YN, Gawade SS, Banerjee SS. Water-powered self-propelled magnetic nanobot for rapid and highly efficient capture of circulating tumor cells. Commun Chem. 2021;4:159.
Zhang Q, Zhou HJ, Chen H, Zhang X, He S, Ma LN, Qu CR, Fang W, Han YJ, Wang D, et al. Hierarchically nanostructured hybrid platform for tumor delineation and image-guided surgery via NIR-II fluorescence and PET bimodal imaging. Small. 2019;15:1903382.
Bourdeau RW, Lee-Gosselin A, Lakshmanan A, Farhadi A, Kumar SR, Nety SP, Shapiro MG. Acoustic reporter genes for noninvasive imaging of microorganisms in mammalian hosts. Nature. 2018;553:86–90.
Tian MG, Ma YY, Lin WY. Fluorescent probes for the visualization of cell viability. Acc Chem Res. 2019;52:2147–57.
Scott JI, Gutkin S, Green O, Thompson EJ, Kitamura T, Shabat D, Vendrell M. A functional chemiluminescent probe for in vivo imaging of natural killer cell activity against tumours. Angew Chem Int Edit. 2021;60:5699–703.
Amin K, Dannenfelser RM. In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95:1173–6.
Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46:4218–44.
Sousa de Almeida M, Susnik E, Drasler B, Taladriz-Blanco P, Petri-Fink A, Rothen-Rutishauser B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem Soc Rev. 2021;50:5397–434.
Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2011;108:18837–42.
Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastro Hepat. 2017;14:455–66.
Huang J, Chen X, Jiang Y, Zhang C, He S, Wang H, Pu K. Renal clearable polyfluorophore nanosensors for early diagnosis of cancer and allograft rejection. Nat Mater. 2022;21:598–607.
Zhao H, Zhao H, Jiao Y, Zhu Y, Liu C, Li F, Wang Y, Gu Z, Yang D. Biosynthetic molecular imaging probe for tumor-targeted dual-modal fluorescence/magnetic resonance imaging. Biomaterials. 2020;256:120220.
Miao L, Lin JQ, Huang YX, Li LX, Delcassian D, Ge YF, Shi YH, Anderson DG. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat Commun. 2020;11:2424.
Funding
We thank the National Natural Science Foundation of China (32260020, 32160038, 32260028, 32460244 and 22206152), Hainan Provincial Natural Science Foundation of China (321RC464, 322MS027), the Education Department of Hainan Province (Hnky2022-8), the Key R&D Program of Hainan Province (ZDYF2024XDNY164), and the Startup Funding of Hainan University (KYQD(ZR)21200).
Author information
Authors and Affiliations
Contributions
J.L., Z.L., Y.C. and D.H. designed the study. Y.C., J. L., X.C. and X.J. performed the experiments. Y.C., J. L., X.J. and X.M. performed the computational analyses. Y.C., J. L., X.J., Y.T. and H.L. analyzed the data. J.L., Z.L., Y.C. and X.J. wrote the paper, and the other authors contributed to the writing. All authors read and approved the final version of the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the animal experiments were approved and conducted in accordance with the guidelines and recommendations from the ethical committee of the Hainan University (Approve number: HNUAUCC-2023-00172).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Cui, Y., Jiang, X. et al. Surface-engineered bio-manufactured gas vesicles for multimodal imaging of glioma. J Nanobiotechnol 23, 116 (2025). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12951-025-03203-6
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12951-025-03203-6