- Research
- Open access
- Published:
Hemoglobin-loaded hollow mesoporous carbon-gold nanocomposites enhance microwave ablation through hypoxia relief
Journal of Nanobiotechnology volume 23, Article number: 326 (2025)
Abstract
Microwave ablation, as a critical minimally invasive technique for tumor treatment, remains challenging in achieving an optimal balance between incomplete and excessive ablation. In addition to selectively elevating the temperature of tumor lesions through the microwave thermal effect, microwave-responsive nanoparticles can also improve the efficacy of single-session ablation by generating reactive oxygen species (ROS) via the microwave dynamic effect, thereby mitigating the thermal damage to normal tissues caused by high temperature. In this study, ultra-small gold nanoparticles anchored hollow mesoporous carbon nanoparticles (HMCNs) are loaded with hemoglobin (Hb) to serve as microwave ablation nano-sensitizers (HMCN/Au@Hb), which will amplify the microwave dynamic effect by alleviating the hypoxic microenvironment of tumors. Upon microwave irradiation, HMCN/Au@Hb not only improves the microwave-thermal conversion efficiency of tumor lesion but also promotes the ROS generation by increasing oxygen content in the hypoxic tumor microenvironment. More importantly, we found that the hypoxia relief will improve the antitumor response and further enhance the clearance of residual tumor after ablation. Nearly complete ablation was achieved in certain tumor-bearing mice, with no recurrence of the primary tumor observed up to 33 days post-ablation. In comparison to traditional microwave ablation, the survival time of the tumor-bearing mice was significantly extended. Therefore, this work presents an innovative ablation sensitization strategy based on the hypoxia relief and provides a nanosensitizer for microwave ablation integrating great microwave-thermal and dynamic effects along with immune modulation capabilities.
Introduction
Microwave ablation, a minimally invasive therapeutic technique, has been widely applied for treating multitype solid tumors, including hepatocellular carcinoma, lung cancer and breast cancer, due to its advantages such as minimal invasiveness, rapid postoperative recovery and high cell-killing efficiency [1, 2, 3]. Traditional microwave ablation relies on high temperatures (above 60 ℃), which are generated by the rapid oscillation and friction of ions and polar molecules within the tumor tissue under microwave irradiation [4, 5]. However, microwave energy often struggles to uniformly cover the entire irregular tumor lesion, which will cause the tumor residual due to the incomplete ablation [6, 7]. In contrast, increasing the ablation temperature or range can cause severe thermal damage to adjacent normal tissues and vital organs owing to heat diffusion and improper distribution [8, 9]. In recent years, a variety of nanoparticles with microwave absorption and heat conversion properties have been developed as sensitization agents of microwave ablation [10, 11, 12, 13]. These include nanocomposites that deliver polar molecules, such as liquid metals and amino acids, as well as dielectric materials, including carbon nanomaterials and transition metal oxides, which significantly enhance local temperatures by selectively improving the microwave absorption and heat conversion efficiency at the tumor site [14, 15, 16, 17, 18, 19, 20]. Unfortunately, tissue carbonization in the ablation “central zone” hinders heat transfer to the marginal “transition zone”, thereby significantly reducing the overall efficacy of the ablation. Moreover, tumor cells in the residual “transition zone”, which undergo sublethal thermal stimulation, are more likely to exhibit aggressive behavior and accelerated growth due to acquired heat tolerance after a single-session ablation [21]. Therefore, developing combination therapy strategies based on multifunctional nanoparticles and exploring their microwave-induced non-thermal effects holds substantial potential for overcoming the limitations of single-session ablation.
Recent studies have found that some microwave-sensitive nanoparticles with specific compositions and structures, such as Fe3O4/Au nanoparticles and metal-organic frameworks (MOFs), can not only convert microwave energy into heat but also generate reactive oxygen species (ROS) under microwave irradiation, exhibiting a phenomenon similar to the photodynamic effect, termed as microwave dynamic effect [5, 22, 23, 24]. Briefly, the interfaces of nanoparticles are typically rich in oxygen vacancies, which induce a mass of free electrons or localized electromagnetic hot spots on the composite material under microwave irradiation, thereby promoting local surface plasmon resonance (LSPR) effect and producing ROS through the interaction of free electrons with oxygen at the nanoparticle interface [17, 22, 25, 26, 27, 28]. The synergy between thermal and non-thermal effects (ROS) enhances the thermal sensitivity of tumor cells, thereby improving ablative efficacy. It is noteworthy that ROS production is critically dependent on local oxygen tensions in microwave dynamic effect, and the intensity of this effect is significantly reduced in the hypoxic tumor microenvironment (TME) with limited oxygen availability [29, 30, 31]. More seriously, the thermal effects of microwave ablation typically disrupt tumor vasculature, blocking blood supply and further exacerbating hypoxic lesions [32]. Therefore, efforts to overcome tumor hypoxia, such as catalyzing in situ oxygen generation, delivering oxygen or enabling microenvironment-activated oxygen release by functional nanoparticles, are expected to enhance the microwave-dynamic effect during ablation [10, 33]. Additionally, hypoxia relief is thought to improve the immunosuppressive TME, thereby enhancing the antitumor immune response following ablation [34, 35].
Herein, ultra-small gold nanoparticles anchored hollow mesoporous carbon nanoparticles (HMCNs) are synthesized as a microwave-absorbing agent and further loaded with hemoglobin (Hb) to develop a nanosensitizer (HMCN/Au@Hb) for microwave ablation, which enhances both the microwave thermal and non-thermal effect (microwave dynamic effect) (Scheme 1a). HMCN/Au@Hb absorbs microwave energy, thereby improving microwave-thermal conversion efficiency at the tumor site. In addition to the thermal effect, HMCN/Au@Hb facilitates electron transfer at the interface and promotes ROS generation by increasing oxygen content in the TME under microwave excitation. Notably, even in the marginal “transition zone”, tumor cell elimination is enhanced through ROS production under low-intensity microwave irradiation. More importantly, HMCN/Au@Hb modulates the immunosuppressive microenvironment and improve the antitumor immune response by relieving tumor hypoxia, thereby enhancing the clearance of residual tumor and inhibiting recurrence after ablation (Scheme 1b). Consequently, this work paves the way for novel strategies to sensitize ablation and provides a promising sensitizer for microwave ablation.
Illustration of synthesis and antitumor mechanism of HMCN/Au@Hb. (a) Synthesis process of HMCN/Au@Hb. Briefly, SiO2 and resorcinol-formaldehyde (RF) oligomers were co-condensed on SiO2 core particles, followed by carbonization under a N2 atmosphere. Subsequently, the silica was etched using NaOH to form hollow mesoporous carbon nanospheres (HMCNs). After anchoring ultra-small gold nanoparticles onto the surface and loading hemoglobin (Hb), the nanocomposites were further functionalized with polyethylene glycol (PEG) to prepare HMCN/Au@Hb. (b) Microwave ablation sensitization employed with HMCN/Au@Hb which integrates great microwave-thermal and dynamic effects along with immune modulation capabilities. HMCN/Au@Hb can effectively absorb microwave energy, leading to the formation of hot spots at the interfaces between HMCN and Au. This phenomenon induces electron transitions and excites trapped oxygen molecules, thereby producing reactive oxygen species (ROS). Meanwhile, HMCN/Au@Hb driven hypoxia relief not only improves ROS production and microwave ablation effect by supplying oxygen, but also synergically enhances the anti-tumor immune effect after ablation by down-regulating the expression of HIF-1α.
Results and discussion
Preparation and characterization of HMCN/Au@Hb
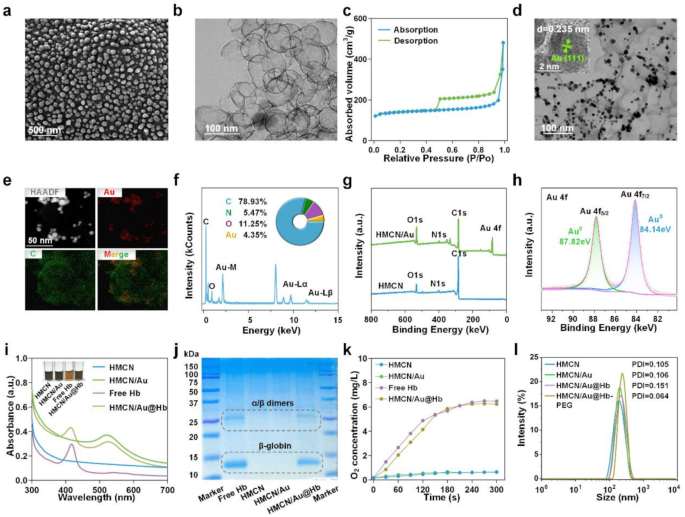
Initially, HMCN was fabricated as a core–shell structure using a modified hard-templating method. Scanning electron microscopy (SEM) images revealed that HMCN were spherical, uniformly distributed, and had an average diameter of 57.06 ± 10.2 nm (Fig. 1a). Transmission electron microscopy (TEM) further demonstrated that the HMCN possessed an inner hollow structure alongside with an average ultrathin shell thickness of 2.35 nm (Fig. 1b). Brunauer Emmet Teller (BET) N2 absorption-desorption analysis confirmed the presence of abundant voids with pore size of 1.76 nm and a high specific surface area up to 532.7475 m2/g, contributing to the enhanced loading capacity of HMCN (Fig. 1c and Figure S1). After physical absorption of Au onto the surface of HMCN, Au presented as protrusions in TEM images, like chocolate beans on cookies. Au and HMCN were thus tightly integrated and HMCN/Au was successfully fabricated (Fig. 1d). Furthermore, the lattice plane spacing of 0.235 nm measured through the high-resolution TEM (HRTEM) image corresponds to the (111) diffraction plane of Au (Fig. 1d insert). Simultaneously, the successful decoration of Au was proved by mapping element profiles (Fig. 1e), and the coexistence of Au and C elements in the HMCN/Au were further validated by energy-dispersive X-ray spectrometer (EDS) (Fig. 1f). Afterwards, X-ray photoelectron spectroscopy (XPS) was performed to measure the element composition and valence states of HMCN/Au (Fig. 1g). The high-resolution Au 4f spectrum can be deconvoluted into two doublets centered at 87.82 and 84.14 eV, labeled as Au4f5/2 and Au4f7/2, respectively, which indicated that Au precursor was partially reduced to metallic Au (Fig. 1h) [36]. In addition, the selected area electron diffraction (SAED) pattern with the (111), (200), (220) and (311) planes of cubic HMCN/Au echo the results from HRTEM (Figure S2).
Preparation and characterization of HMCN/Au@Hb. (a) SEM and (b) TEM images of HMCN. (c) N2 absorption/desorption isotherms of HMCN. (d) TEM images of HMCN/Au (Insert was HRTEM). (e) HAADF-STEM image and elemental mapping for HMCN/Au. (f) Quantitative elemental analysis of HMCN/Au conducted using EDS. The inserts were the comprised element and corresponding atomic frequencies. (g) XPS analysis of the HMCN and HMCN/Au. (h) The high-resolution XPS spectra of Au 4f for HMCN/Au. (i) UV-VIS spectra of free Hb, HMCN, HMCN/Au and HMCN/Au@Hb, respectively (Insert was the color change before and after loading). (j) Coomassie blue staining performed on electrophoresed acrylamide loaded with Hb. (k) Oxygen releasing properties measured by RDPP probe. (l) Size distribution of HMCN, HMCN/Au, HMCN/Au@Hb and PEGylated HMCN/Au@Hb determined by DLS.
Given to the hollow and mesopores of HMCN/Au, Hb was efficiently loaded and HMCN/Au@Hb was eventually formed. The UV-VIS spectra of HMCN/Au@Hb exhibited characteristic absorption peaks at 410 and 525 nm corresponding to Hb and Au (Fig. 1i). Moreover, the insert image illustrates the color changes during the synthesis of HMCN/Au@Hb, the dark gray HMCNs turned into a slightly brownish dark grey color after loaded with Hb (Fig. 1i insert). Coomassie blue staining revealed protein bands at 14 and 25 kDa, in correlation with the β-globin and α/β dimers of Hb (Fig. 1j) [37]. Based on the standard concentration curve of Hb (Figure S3a, b), the optimal mass ratio was determined to be 1:5 (Hb: HMCN) (Table S1). The oxygen-carrying capacity of different nanomaterials was then assessed using a dissolved-oxygen meter. In particular, a significant rise in O2 levels was detected in free Hb and HMCN/Au@Hb, which confirmed that Hb retained its oxygen-carrying activity during the synthesis process (Fig. 1k). Moreover, the surface of HMCN/Au@Hb were modified with DSPE-PEG2000-NH2 to enhance the biocompatibility and stability in physiological condition. Dynamic light scattering (DLS) analysis showed a slightly increased particle size following Au decoration, Hb loading and PEGylation. The hydrodynamic sizes of HMCN, HMCN/Au, HMCN/Au@Hb and PEGylated HMCN/Au@Hb dispersed in deionized water were measured as 177.57 ± 5.10 nm (PDI = 0.105), 205.50 ± 2.50 nm (PDI = 0.106), 228.67 ± 3.05 nm (PDI = 0.151) and 259.77 ± 4.95 nm (PDI = 0.064), respectively (Fig. 1l), with a uniform size distribution. In summary, the successful preparation of HMCN/Au@Hb was confirmed through element composition determination, morphological observation and spectral characterization.
Microwave responsive and oxygen carrying capacities of HMCN/Au@Hb
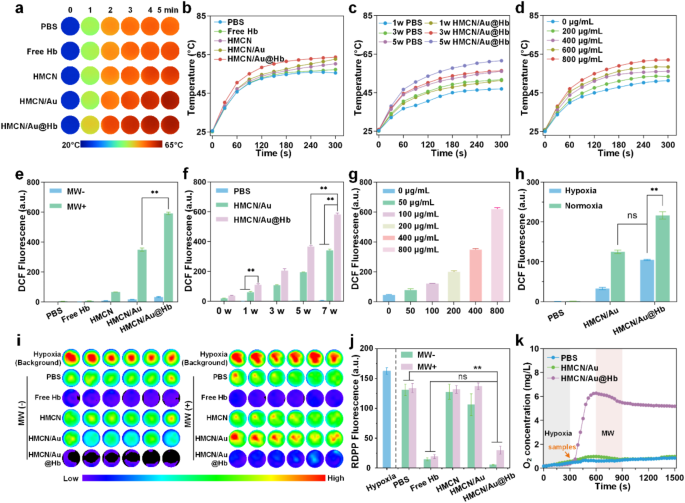
Considering HMCN exhibits high dielectric constants due to its unique porous structure and conductive carbon framework, the microwave-thermal performance of HMCN/Au@Hb was monitored under controlled microwave parameters, including power density and nanocomposites concentration [38]. Following a 5-min microwave irradiation (3.0 W), the control and free Hb group only increased to 55.5 and 57.2℃, respectively. While under the same irradiation condition, HMCN, HMCN/Au and HMCN/Au@Hb (400 µg/mL) were heated up to 60.2, 62.6 and 63.7℃, respectively, the final temperature of HMCN/Au@Hb was about 8.2℃ higher than the temperature of PBS. These results demonstrate that HMCN/Au@Hb contributed to enhancing microwave-to-heat conversion efficiency to a certain extent (Fig. 2a) (corresponding infrared thermal image was presented in Fig. 2b). Additionally, HMCN/Au@Hb was irradiated with microwave under different power densities, the temperature and the microwave power density were positively correlated (Fig. 2c) (corresponding infrared thermal image was presented in Figure S4a). Moreover, upon exposure to microwave (3.0 W), the temperature increased from 51.3 to 53.4, 56.1, 58.4 and 62.0℃ with increasing concentration of HMCN/Au@Hb (0, 200, 400, 600 and 800 µg/mL) (Fig. 2d), indicating that the microwave-thermal effect of HMCN/Au@Hb was proportional to the sample concentration (corresponding infrared thermal image was presented in Figure S4b). These findings highlight the potential of HMCN/Au@Hb as an excellent microwave absorption agent to improve the microwave-thermal conversion efficiency for tumors.
Microwave responsive and oxygen carrying capacities of HMCN/Au@Hb. (a) Real-time infrared thermal images and (b) corresponding heating profiles of different samples (400 µg/mL) under microwave irradiation (3 W, 5 min). (c) Microwave heating profiles of PBS and HMCN/Au@Hb (400 µg/mL) under microwave irradiation at different power densities for 5 min. (d) Microwave heating profiles of HMCN/Au@Hb at different concentrations under microwave irradiation (3 W, 5 min). (e) ROS detection of different samples (200 µg/mL) irrespective of microwave irradiation (7 W, 5 min). (f) ROS detection of different samples (200 µg/mL) under microwave irradiation (5 min) at different power levels. (g) ROS detection of HMCN/Au@Hb suspensions at different concentrations under microwave irradiation (3 W, 5 min). (h) ROS detection of different samples (PBS, HMCN/Au and HMCN/Au@Hb) at the concentration of 200 µg/mL under hypoxic or normoxic conditions under microwave irradiation (3 W, 5 min). (i) Fluorescence images and (j) quantitative analyses of different samples showing oxygen generation under different conditions. (k) Dynamic oxygen generation of different samples under microwave irradiation (3 W, 5 min). Data were presented as mean values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Inspired by the strong SPR characteristic of Au and the sufficient oxygen released by Hb, we inferred that Au could generate active or “hot” electrons so as to induce electron’s transition upon microwave irradiation and further absorb oxygen molecules as sustainable fuel for ROS generation [39]. To investigate whether the HMCN/Au@Hb possessed the ability of microwave-dynamic performance, the production and species determination of ROS under microwave irradiation were performed through electron spin resonance (ESR). Notably, the HMCN/Au@Hb + MW group exhibited a characteristic sextet signal corresponding to superoxide anions (•O2−), which was remarkably higher than the HMCN/Au + MW group. The result indicated that Hb might play a vital role in oxygen-amplified microwave-dynamic performance (Figure S5). We then used the DCFH-DA probe to detect total ROS generation of HMCN/Au@Hb. Upon microwave irradiation (7.0 W), the fluorescence intensity of HMCN/Au@Hb was almost 1.7 times higher than that of HMCN/Au, indicating the positive effect of oxygen in amplifying ROS generation (Fig. 2e). Subsequently, the fluorescence intensity of PBS, HMCN/Au and HMCN/Au@Hb showed a similarly power-dependent increase. Under the same power density, the HMCN/Au@Hb group exhibited greater fluorescence intensity compared to that of the HMCN/Au group (Fig. 2f). Moreover, the ROS generation of HMCN/Au@Hb showed a concentration-dependent trend (Fig. 2g). Therefore, we believed that the oxygen carried by HMCN/Au@Hb facilitated the amplification of ROS generation. After irradiated with 3.0 W microwave, the ROS signals of HMCN/Au in normoxic environment was 1.7 times higher than that in hypoxic environment, but comparable to HMCN/Au@Hb in the same hypoxic environment (Fig. 2h). The result demonstrated that Hb might supplement the O2 content in hypoxic environment, which would further facilitate the oxidation process.
To further explore the inner oxygen-carrying property of HMCN/Au@Hb, we employed an oxygen indicator, RDPP probe, which can be quenched by molecular oxygen, to visually investigate the oxygen production [40]. Notably, the fluorescence intensity in the PBS and HMCN groups displayed a negligible change. However, the fluorescence intensity in both the free Hb and HMCN/Au@Hb group weakened dramatically, suggesting the similarly superior O2 generation activities of both the free Hb and HMCN/Au@Hb (Fig. 2i), which was consistent with the corresponding quantitative data presented in Fig. 2j. Besides, both PBS and HMCN/Au contained very little oxygen and remained unchanged after irradiating with microwave, whereas HMCN/Au@Hb (3 mg/mL) showed immediate increase in the O2 concentration at a hypoxic solution and maintained the extremely high level of O2 during the process of microwave irradiation (Fig. 2k). The above results confirmed the oxygen-carrying activity of HMCN/Au@Hb, as well as a small amount of oxygen consumption to generate ROS under the exposure of microwave.
Cytotoxicity of HMCN/Au@Hb under microwave irradiation
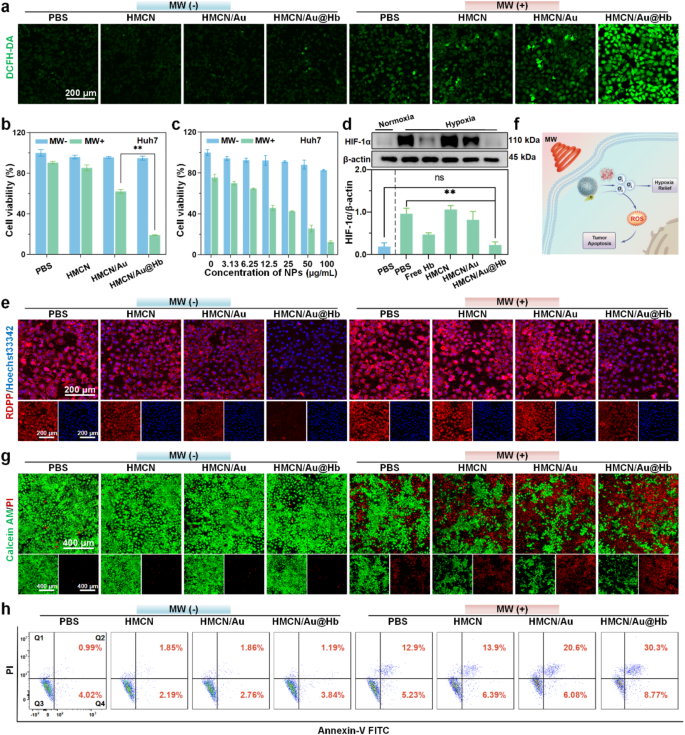
Inspired by the ROS generation property at the solution level, we further evaluated HMCN/Au@Hb induced ROS outbreak at the cellular level. DCFH-DA probe was utilized to visualize and characterize the ROS generation via confocal laser scanning microscope (CLSM) [41]. Herein, invisible green fluorescence was detected in cells treated with PBS or NPs alone, while more intense green fluorescence was shown upon microwave irradiation. Moreover, the brightest green fluorescence was observed in the HMCN/Au@Hb + MW group (Fig. 3a). Furthermore, the quantitative data across different groups found that the ROS levels in the HMCN/Au@Hb + MW group were approximately 1.8-fold higher than that of the HMCN/Au + MW group, indicating that intrinsic oxygen-carrying capacity of Hb might induce a greatly enhanced microwave-dynamic effect (Figure S6a). Subsequently, the aforementioned cells were harvested for flow cytometry analysis, and showed a consistent trend compared to those observed in DCFH-DA assay (Figure S6b). These findings directly highlighted the effectiveness of HMCN/Au@Hb in generating ROS triggered by microwave irradiation. We further utilized upper transwell chamber to mimic “transition zone” to investigate whether tumor cells in the marginal “transition zone” could still cause ROS burst under ultra-low microwave irradiation, Although the temperature in the upper chamber of the microwave irradiation group was significantly lower than that in the bottom chamber and direct heating group, the ROS levels were comparable to those in the direct heating group. It further demonstrated that even under low-power microwave irradiation in the “transition zone”, nanoparticles could still be effectively excited to produce ROS (Figure S7).
In vitro microwave-induced cytotoxic effect. (a) CLSM images of intracellular ROS level with different treatments (green, fluorescence of DCFH-DA). (b) Cytotoxicity of Huh-7 cells treated with different samples and (c) treated with HMCN/Au@Hb at different concentrations. (d) Western blot demonstrating the HIF-1α expression levels with different treatments. (e) CLSM images of intracellular O2 level with different treatments (blue, fluorescence of Hoechst 33342; red, fluorescence of RDPP; overlay images). (f) Schematic illustration of HMCN/Au@Hb mediated microwave thermal-dynamic effect. (g) Live/Dead co-staining and (h) Annexin V/PI staining of Huh-7 cells after different treatments. Data were presented as mean values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Given that excessive oxidative stress detrimentally impacts the cellular killing effect, the antitumor effect of HMCN/Au@Hb in Huh-7 cells was initially characterized by MTT assay [42]. After being irradiated with microwave, the cell viability rate reveals an approximately 80% reduction in MW + HMCN/Au@Hb (50 µg/mL) group, which was higher than that in MW + HMCN/Au (50 µg/mL) group (Fig. 3b). Especially, cell viability was gradually reduced in the HMCN/Au@Hb + MW group with increasing concentrations of HMCN/Au@Hb, supporting its effectively antitumor effect in a concentration-dependent manner. By contrast, insignificant cytotoxic effect was observed in non-irradiated conditions, suggesting the noncytotoxic nature of HMCN/Au@Hb (Fig. 3c). To further investigate the cytotoxicity of HMCN/Au@Hb in normal cell lines, human normal hepatic cell (LO2 and MIHA) and renal cell (293T) were used in the following experiments. Even at an extremely high concentration of 400 µg/mL, 3 types of cell line remained viability of 76.4%, 85.6% and 80.7%, respectively, indicating the commendable biosafety of HMCN/Au@Hb (Figure S8).
Due to the sufficient O2 released by HMCN/Au@Hb, we speculated that the inherent oxygen levels would substantially elevate and mitigate the constraints imposed by the hypoxic microenvironment, thereby facilitating ROS production and amplifying the therapeutic effect [43]. Preliminary investigation of the hypoxia-relieving effect in vitro, western blot was conducted to detect the expression level of HIF-1α [44]. HIF-1α expression level was significantly down-regulated in the HMCN/Au@Hb group but comparable to that of the negative control (PBS under normoxic conditions), solidly verifying that both Hb and HMCN/Au@Hb possessed equal hypoxia-allaying effects (Fig. 3d). Subsequently, to investigate the dynamic process of oxygen variation, cellular O2 content was analyzed by CLSM observation using the RDPP indicator. After incubated with HMCN/Au@Hb, the red fluorescence intensity was weakened acutely compared to that of the PBS group, suggesting a substantial increase in cellular oxygen levels. Nevertheless, the red fluorescence in the HMCN/Au@Hb + MW group was slightly stronger than that in the HMCN/Au@Hb group (Fig. 3e). Fluorescence semi-quantitative (Figure S9a) and flow cytometry measurements (Figure S9b) further confirmed that oxygen consumption may occur through generating ROS upon microwave stimulus. Altogether, owing to the specific hypoxic nature of tumor tissue, we speculated that the sufficient oxygen released by Hb-loaded HMCN/Au might facilitate ROS generation in vitro, thereby ameliorating the hypoxic microenvironment and augmenting the therapeutic efficacy (Fig. 3f) [45].
In addition, the antitumor effect induced by excessive ROS and microwave hyperthermia was assessed using Calcein-AM/PI co-staining. Ultralow cell death rates were observed in non-irradiated groups. After irradiation, cell death rates increased dramatically, and cells incubated with HMCN/Au@Hb exhibited the highest death rate, which was conformed to the findings of the MTT assay (Fig. 3g). The fluorescence quantitative results also remarkably emphasized the significant outcomes of the MW coupling system (Figure. S10). Consequently, cell apoptosis assay was performed on Huh-7 cells to identify the type of apoptosis triggered by ROS and hyperthermia. Cells treated with HMCN/Au + MW and HMCN/Au@Hb + MW displayed a substantial increase in the proportion of apoptotic (Annexin V-positive) and necroptotic cells (PI-positive) (Fig. 3h). Especially, obvious apoptosis signals (Q2 + Q4 quadrant: 39.1%) were observed in the HMCN/Au@Hb + MW group, which was superior to that of the HMCN/Au + MW group (Q2 + Q4 quadrant: 26.7%) (Figure. S11).
In vivo antitumor effect
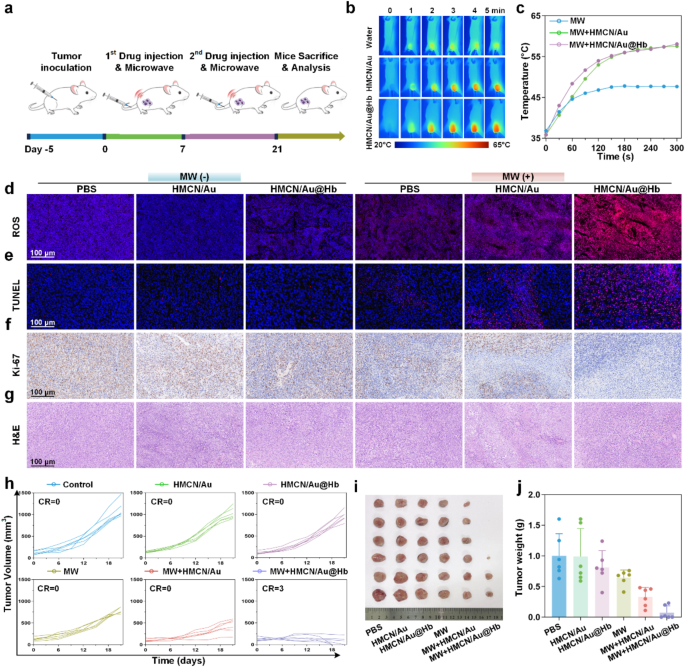
Due to the remarkable in vitro cell death effect, the in vivo antitumor effects were evaluated on tumor-bearing mice. The HCC models were randomly divided into six groups (n = 6): PBS, HMCN/Au, HMCN/Au@Hb and their respective MW groups. Figure 4a showed the entire in vivo experimental process. Throughout the treatment, in vivo infrared thermal images (Fig. 4b) and corresponding temperature curves (Fig. 4c) revealed that tumor temperature in the MW + HMCN/Au and MW + HMCN/Au@Hb groups increased rapidly within 5 min, even reached 58.1 °C and 57.6 °C under an ultralow microwave power density (3.0 W), respectively. However, the temperature of tumors without NPs administration only increased to 47.7 °C, indicating that HMCN/Au and HMCN/Au@Hb exhibited an excellent microwave-thermal effect in vivo due to their similar localized heat accumulation ability. To further evaluate the biodistribution of HMCN/Au@Hb, ICG labeled fluorescence imaging was performed on Hepa1-6 tumor xenograft mice. Following intravenous injection of free ICG or HMCN/Au@Hb@ICG, fluorescence images were acquired at different timepoints (0, 1, 2, 4, 6, 8, 12 and 24 h). Both groups exhibited strong fluorescence signal at 1 h (Figure S12a). Notably, HMCN/Au@Hb demonstrated significantly higher fluorescence retention at the tumor site compared to free ICG, with stable tumor fluorescence persisting beyond 24 h, whereas the ICG group showed minimal signal beyond 8 h. This prolonged tumor accumulation was likely attributed to the optimal nanoparticle size, facilitating the enhanced permeability and retention (EPR) effect (Figure S12b). Ex vivo imaging further confirmed sustained fluorescence in tumors, supporting the prolonged retention of HMCN/Au@Hb in tumor tissue (Figure S12c). In addition, we supplemented the Au content in tumor and other organs with Inductively Coupled Plasma Mass Spectrometry (ICP-MS), it revealed that obvious Au enrichment in the tumor of HMCN/Au@Hb group at the time of sacrifice (1-hour post-injection), demonstrating the excellent tumor accumulation potential. Besides, it also demonstrated significant hepatic and renal uptake, presumably due to the primary involvement of the liver and kidneys in the metabolic clearance of the nanomaterials (Figure S13). However, ex vivo distribution of both free ICG and ICG-labeled HMCN/Au@Hb decreased rapidly and was nearly completely cleared after 24 h administration, as previously shown in Figure S12c.
In vivo antitumor effect. (a) Schematic illustration of the experimental schedule for the subcutaneous tumor model treatments. (b) Infrared thermal images and (c) the corresponding heating profiles of tumor-bearing mice during microwave irradiation. Histological analysis of sacrificed tumor tissues after a 21-day treatment (d) ROS, (e) TUNEL, (f) Ki-67 and (g) H&E staining. (h) Individual of tumor growth curves in different groups. (i) Photographs (j) and the corresponding weight of tumor tissues at 21st day after treatments. Data were presented as mean values ± SD (n = 6). (* represents p<0.05, ** represents p<0.01)
The potent antitumor capability of HMCN/Au@Hb was further investigated through ROS, TUNEL, Ki-67 and H&E staining of tumor tissues dissociated on day 21. Significant variations in the morphology of tumors were observed across different treatment groups. Remarkably, HMCN/Au@Hb + MW treated tumors exhibited higher levels of ROS generation than the other groups, indicating that ROS outbreak may play a pivotal role in tumor suppression (Fig. 4d, Figure S14a). In addition, TUNEL staining showed that HMCN/Au@Hb + MW group exhibited the most intense red fluorescence, indicative of apoptotic tumor cell, highlighting the superior microwave-dynamic property to induce cancer cell apoptosis (Fig. 4e, Figure S14b). Similarly, tumors stained with Ki-67 (Fig. 4f, Figure S14c) and H&E (Fig. 4g) revealed that HMCN/Au@Hb + MW displayed the lowest tumor cell proliferation and the most extensive karyorrhectic debris, in sharp contrast to the abundant proliferation and limited necrosis observed in other groups. These findings demonstrated that HMCN/Au@Hb achieved pronounced antitumor effect by inducing ROS outbreak upon microwave irradiation.
Encouraged by the great performance of HMCN/Au@Hb in thermal imaging and tumor cell damage in vivo, changes in tumor volume were monitored throughout 21-day treatment (Figure S15). Compared to the rapidly tumor growth in the control, HMCN/Au, HMCN/Au@Hb and MW-only groups, only moderate tumor inhibition observed in the HMCN/Au + MW group, while the tumor growth curves began to increase starting on the 15th day, since the insufficient oxygen may produce inadequate microwave-dynamic effect and ultimately lead to incomplete tumor necrosis and recurrence. Meanwhile, the HMCN/Au@Hb + MW group presented with substantial tumor suppression, as well as a significant and definitive curative effect (Fig. 4h and Figure S16). Tumor photographs (Fig. 4i) and tumor weights (Fig. 4j) explained the similar result in a more intuitive and clear way. Furthermore, the potential side effects were evaluated during the 21-day observation period. Notably, no significant fluctuations were observed in body weight across all diverse groups, which preliminarily confirmed the excellent biosafety of all treatments in mice (Figure S17). To further investigate potential thermal damage to normal tissues adjacent to tumor lesions post-ablation, we conducted pathological examinations. Histological analyses revealed a well-demarcated ablation zone, and there were no obvious heating damage effects in surrounding muscle tissues upon microwave irradiation (Figure S18). Moreover, H&E staining was performed on major organs across different treated mice, and no observable tissue necrosis was found. It suggested that both nanomaterials administration and microwave irradiation had no visible pathological abnormalities or inflammatory lesions in vivo (Figure S19). Collectively, HMCN/Au@Hb exhibited superior tumoricidal effect and favorable biosafety, which was expected to be a biocompatible nanomaterial for microwave ablative therapy in the future.
To investigate the tumor recurrence, we further conducted a various of experiment groups to continuously monitor the tumor growth for 33 days (Note: tumor volume ≥ 1500 mm3 defined as dead). Comparative analysis at day 7 post-ablation revealed that the MW + HMCN/Au@Hb group (V/V0 = 0.998) showed better tumor inhibition effect than the MW-alone group (V/V0 = 1.555). Notably, some selected tumor-bearing mice even achieved complete tumor ablation in MW + HMCN/Au@Hb group. In contrast, the tumor volume in the non-ablation group surpassed the ethical endpoint threshold (≥ 1500 mm3), necessitating termination of observation due to rapid progression (Figure S20a). More encouragingly, there was no significant local recurrence in the MW + HMCN/Au@Hb group even at day 33 post-ablation (V/V0 = 0.414), in sharp contrast to the MW group (V/V0 = 7.481) (Figure S20b). In addition, the survival rate of mice treated with HMCN/Au@Hb plus MW was significantly prolonged (Figure S20c). Therefore, compared to microwave ablation alone, HMCN/Au@Hb significantly enhanced the efficacy of microwave ablation and effectively inhibited tumor recurrence at post-ablation.
Antitumor immune response
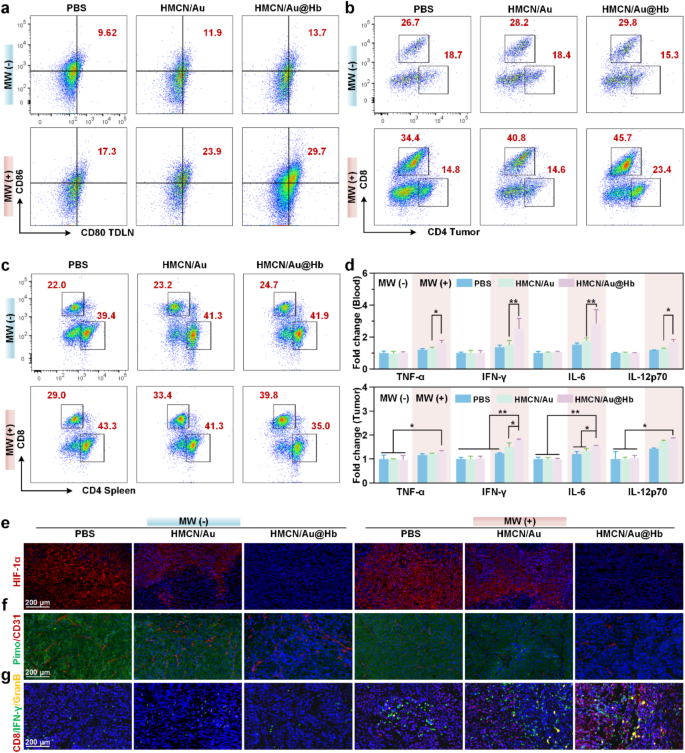
To further investigate the underlying mechanism of the antitumor effect of HMCN/Au@Hb, the activation of systemic immunity was explored [46]. Previous studies have shown that DCs, mostly contained in tumor draining lymph nodes (TDLNs), acted as potent antigen-presenting cells (APCs) for capturing tumor associated antigens (TAAs) [47]. Herein, we found that the HMCN/Au@Hb + MW group showed a greater matured DCs (CD80+CD86+) infiltration, which far exceeded that observed in the HMCN/Au + MW group (Fig. 5a). Quantitative data further confirmed these findings (Figure S21a). The results implied that Hb-loaded HMCN/Au mediated microwave-dynamic effect appeared to facilitate antigen presentation to DCs and trigger downstream immune response. Given to its important role in promoting DC maturation, the differentiation of T lymphocytes within the primary tumor were examined [48]. Notably, the greater CD8+ T cells infiltration were evoked by microwave stimulus and HMCN/Au@Hb administration (Fig. 5b), and the higher intratumoral CD8+/CD3+ T cell ratios were observed in irradiated groups compared with non-irradiated groups, particularly in combination with HMCN/Au@Hb (Figure S21b). Next, we performed flow cytometry analysis in splenic homogenates of mice to investigate the activation of T cells in immune organs. The percentage of CD8+ T cells were significantly elevated in the HMCN/Au@Hb + MW group compared to the other groups (Fig. 5c). Likewise, higher CD8+/CD3+ T cell ratio were observed in the HMCN/Au@Hb + MW group than that of the HMCN/Au + MW group (Figure S21c). These findings suggested that the oxygen-amplified microwave-dynamic effect enabled infiltration of immune cells to a larger extent.
Antitumor immune response. (a) Flow cytometry of DC maturation in TDLNs with indicated treatments. (b) Flow cytometry of T-cell activation in tumors and (c) spleens with indicated treatments. (d) ELISA analysis of the TNF-α, IFN-γ, IL-6, and IL-12p70 levels in serum and tumors. Immunofluorescence images of (e) HIF-1α, (f) Pimonidazole and CD31+, (g) CD8, IFN-γ and Granzyme B exposure in tumors. Data were all presented as mean values ± SD (n = 3). (* represents p<0.05, ** represents p<0.01)
Moreover, the role of various cell-secreted pro-inflammatory factors, such as TNF-𝛼, IFN-𝛾, IL-6, and IL-12p70, all of which had a vital function in modulating immune responses, were further explored via ELISA assay [49]. ELISA assay portrayed an elevated serum secretion levels of 1.28-, 1.68-, 1.61- and 1.33-fold for TNF-𝛼, IFN-𝛾, IL-6 and IL-12p70 in HMCN/Au@Hb + MW group than that in the HMCN/Au + MW group, respectively. As for intratumoral secretion levels, HMCN/Au@Hb upon microwave irradiation yielded the greatest TNF-𝛼, IFN-𝛾, IL-6 and IL-12p70 secretions, whereas their respective secretions in other groups were relatively lower, confirming that HMCN/Au@Hb plus MW could exert a robust activated-inflammatory effect (Fig. 5d).
Aiming to deepen our comprehension of the interaction between hypoxic relief and intrinsic antitumor immunity of HMCN/Au@Hb in vivo, immunofluorescence experiments were then performed on mouse tumor tissues. The specific hypoxic status of tumors was evaluated by HIF-1α expression levels [50]. Apparently, a significant down-regulation was observed in the HMCN/Au@Hb + MW group compared to the HMCN/Au + MW group (Fig. 5e, Figure S22). Meanwhile, accumulating evidence has shown that insufficient microwave treatment could aggravate hypoxia and induce angiogenesis in tumor sites, pimodazole and CD31+ staining were adopted for detecting hypoxic regions and microvessels [51]. Compared with the MW group, a dramatically reduction of green fluorescence (pimodazole staining) and red fluorescence (CD31+ staining) were observed in the HMCN/Au@Hb + MW group (Fig. 5f, Figure S22). It suggested the improved oxygenation and less angiogenesis at the tumor site, which would potentially contribute to reversing the immune-suppressive TME. Immunofluorescence analysis for CD8+, IFN-γ and Granzyme B further verified the increased infiltration of effector cytotoxic CD8+ T lymphocytes in tumor sites [47]. CD8+, IFN-γ and Granzyme B infiltration in combination therapy groups were greater than that of the monotherapy groups, and the HMCN/Au@Hb + MW group showed the strongest fluorescence intense (Fig. 5g, Figure S22). These findings confirmed the efficiency of HMCN/Au@Hb in converting naïve T cells into effector T cells (CD8+, IFN-γ and Granzyme B). Overall, the above findings demonstrated that intrinsic oxygen-carrying capacity of Hb induced a greatly enhanced microwave-dynamic effect. Altogether, HMCN/Au@Hb had the unique ability of in situ oxygen generation and maintain in vivo hypoxia-relieving effect, which benefits for reversing the immune-suppressive microenvironment. This study could pave the way towards expediting tumoricidal immunity.
Conclusions
Herein, we prepared a microwave-responsive nanocomposites (HMCN/Au@Hb) to strengthen microwave ablation efficacy by improving the microwave-induced thermal and non-thermal effect (microwave dynamic effect). HMCN/Au@Hb could selectively elevate the temperature of tumor lesions due to its high microwave absorption efficiency and thermal conversion performance. Moreover, we found that under the microwave irradiation, it enhanced the microwave dynamic effect by inducing electron transfer at the interface and supplementing oxygen, thereby promoting the ROS production. In addition, it effectively relieved the tumor hypoxia and down-regulated HIF-1α expression to further improve antitumor immune response after ablation. Therefore, our work is expected to offer a versatile sensitization strategy for microwave ablation and holds significant potential for the treatment of deep-seated hypoxic tumors in clinical settings. Given that microwave ablation is a minimally invasive therapy, future studies could explore more precise drug delivery strategies—such as superselective intra-arterial chemotherapy or other local injection approaches—to enhance the tumor-targeting capability and therapeutic efficacy of our nanoplatform. Furthermore, owing to its porous structure, HMCN/Au@Hb can be loaded with chemotherapeutic agents and immunoadjuvants (e.g., CpG oligonucleotides, PD-1 antibodies, and STING agonists), enabling combinational therapy and potentially boosting post-ablation antitumor immunity and overall treatment outcomes.
Data availability
No datasets were generated or analysed during the current study.
References
Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aube C, Digklia A, Pache I, Deltenre P, Knebel JF, Denys A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–25.
Li L, Zhang X, Zhou J, Zhang L, Xue J, Tao W. Non-Invasive thermal therapy for tissue engineering and regenerative medicine. Small. 2022;18:e2107705.
Cao XJ, Wang SR, Che Y, Liu J, Cong ZB, He JF, Wang HL, Liu G, Guo JQ, Hao Y, et al. Efficacy and safety of thermal ablation for treatment of solitary T1N0M0 papillary thyroid carcinoma: A multicenter retrospective study. Radiology. 2021;300:209–16.
Zhou H, Fu C, Chen X, Tan L, Yu J, Wu Q, Su L, Huang Z, Cao F, Ren X, et al. Mitochondria-targeted zirconium metal-organic frameworks for enhancing the efficacy of microwave thermal therapy against tumors. Biomater Sci. 2018;6:1535–45.
Ma X, Ren X, Guo X, Fu C, Wu Q, Tan L, Li H, Zhang W, Chen X, Zhong H, Meng X. Multifunctional iron-based Metal-Organic framework as biodegradable nanozyme for microwave enhancing dynamic therapy. Biomaterials. 2019;214:119223.
Vogl TJ, Basten LM, Nour-Eldin NA, Kaltenbach B, Bodelle B, Wichmann JL, Ackermann H, Naguib NNN. Evaluation of microwave ablation of liver malignancy with enabled constant Spatial energy control to achieve a predictable spherical ablation zone. Int J Hyperth. 2018;34:492–500.
Qin S, Liu GJ, Huang M, Huang J, Luo Y, Wen Y, Wang Y, Chen L. The local efficacy and influencing factors of ultrasound-guided percutaneous microwave ablation in colorectal liver metastases: a review of a 4-year experience at a single center. Int J Hyperth. 2019;36:36–43.
Zhang Y, Guo L, Kong F, Duan L, Li H, Fang C, Zhang K. Nanobiotechnology-enabled energy utilization elevation for augmenting minimally-invasive and noninvasive oncology thermal ablation. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1733.
Wen L, Liu H, Hu C, Wei Z, Meng Y, Lu C, Su Y, Lu L, Liang H, Xu Q, Zhan M. Thermoacoustic Imaging-Guided Thermo-Chemotherapy for hepatocellular carcinoma sensitized by a Microwave-Responsive nitric oxide nanogenerator. ACS Appl Mater Interfaces. 2023;15:10477–91.
Zhang W, Zhou H, Gong D, Wu H, Huang X, Miao Z, Peng H, Zha Z. AIPH-Encapsulated Thermo-Sensitive liposomes for synergistic microwave ablation and Oxygen-Independent dynamic therapy. Adv Healthc Mater. 2023;12:e2202947.
Shi H, Liu T, Fu C, Li L, Tan L, Wang J, Ren X, Ren J, Wang J, Meng X. Insights into a microwave susceptible agent for minimally invasive microwave tumor thermal therapy. Biomaterials. 2015;44:91–102.
Long D, Liu T, Tan L, Shi H, Liang P, Tang S, Wu Q, Yu J, Dou J, Meng X. Multisynergistic platform for tumor therapy by mild microwave Irradiation-Activated chemotherapy and enhanced ablation. ACS Nano. 2016;10:9516–28.
Fu C, Zhou H, Tan L, Huang Z, Wu Q, Ren X, Ren J, Meng X. Microwave-Activated Mn-Doped zirconium Metal-Organic framework nanocubes for highly effective combination of microwave dynamic and thermal therapies against Cancer. ACS Nano. 2018;12:2201–10.
Wei H, Tian Y, Chen Q, Estevez D, Xu P, Peng H-X, Qin F. Microwave absorption performance of 2D Iron-Quinoid MOF. Chem Eng J. 2021; 405.
Li R, Tian Y, Zhu B, Wang Y, Dang R, Zhao L, Yang S, Li Y, Wen N. Graphene-containing metal-organic framework nanocomposites for enhanced microwave ablation of salivary adenoid cystic carcinoma. Nanoscale Adv. 2022;4:1308–17.
Blackburn JL, Ferguson AJ, Cho C, Grunlan JC. Carbon-Nanotube-Based thermoelectric materials and devices. Adv Mater. 2018; 30.
Zhu H, Li B, Liu X, Qiao Y, Lv Y, Zheng Y, Zhu S, Li Z, Cui Z, Shen J, Wu S. Interfacial Mo, W-Conjugated polarization, and oxygen vacancies of MoO2/WO3 in enhanced microwave therapy for MRSA-Induced osteomyelitis. ACS Nano. 2022;16:21098–110.
Mao C, Jin W, Xiang Y, Zhu Y, Wu J, Liu X, Wu S, Zheng Y, Cheung KMC, Yeung KWK. Reversing Multidrug-Resistant Escherichia coli by compromising its BAM biogenesis and enzymatic catalysis through microwave hyperthermia therapy. Adv Funct Mater. 2022; 32.
Chen Y, Xu P, Wu M, Meng Q, Chen H, Shu Z, Wang J, Zhang L, Li Y, Shi J. Colloidal RBC-shaped, hydrophilic, and Hollow mesoporous carbon nanocapsules for highly efficient biomedical engineering. Adv Mater. 2014;26:4294–301.
Qiao Y, Liu X, Li B, Han Y, Zheng Y, Yeung KWK, Li C, Cui Z, Liang Y, Li Z, et al. Treatment of MRSA-infected osteomyelitis using bacterial capturing, magnetically targeted composites with microwave-assisted bacterial killing. Nat Commun. 2020;11:4446.
Wu S, Zhang D, Yu J, Dou J, Li X, Mu M, Liang P. Chemotherapeutic Nanoparticle-Based liposomes enhance the efficiency of mild microwave ablation in hepatocellular carcinoma therapy. Front Pharmacol. 2020;11:85.
Fu J, Li Y, Zhang Y, Liang Y, Zheng Y, Li Z, Zhu S, Li C, Cui Z, Wu S. An engineered Pseudo-Macrophage for rapid treatment of Bacteria-Infected osteomyelitis via Microwave-Excited Anti-Infection and immunoregulation. Adv Mater. 2021;33:e2102926.
Fernandes DA. Multifunctional gold nanoparticles for cancer theranostics. 3 Biotech. 2024;14(11):267.
Fernandes DA. Review on Metal-Based theranostic nanoparticles for Cancer therapy and imaging. Technol Cancer Res Treat. 2023;22.
Wu Q, Yu Y, Yu X, Du Q, Gou L, Tan L, Fu C, Ren X, Ren J, Xiao K, Meng X. Engineering liquid metal-based nanozyme for enhancing microwave dynamic therapy in breast cancer PDX model. J Nanobiotechnol. 2023;21:399.
Wang Y, Ren X, Zheng Y, Tan L, Li B, Fu C, Wu Q, Chen Z, Ren J, Yang D, et al. Boosting microwave Thermo-Dynamic Cancer therapy of TiMOF via COF-Coating. Small. 2023;19:e2304440.
Sun W, Wang C, Wan D, Zheng Y, Wu S, Shen J, Zhang Y, Liu X. CuCeO bimetallic oxide rapidly treats Staphylococcus aureus-Infected osteomyelitis through microwave strengthened microwave catalysis and Fenton-Therapy. Small Methods. 2023;7:e2300203.
Jin L, Zheng Y, Liu X, Zhang Y, Li Z, Liang Y, Zhu S, Jiang H, Cui Z, Wu S. Magnetic composite rapidly treats Staphylococcus aureus-Infected osteomyelitis through microwave strengthened thermal effects and reactive oxygen species. Small. 2022;18:e2204028.
Fu J, Li T, Zhu Y, Hao Y. Ultrasound-Activated oxygen and ROS generation nanosystem systematically modulates tumor microenvironment and sensitizes sonodynamic therapy for hypoxic solid tumors. Adv Funct Mater. 2019; 29.
Guo X, Qu J, Zhu C, Li W, Luo L, Yang J, Yin X, Li Q, Du Y, Chen D, et al. Synchronous delivery of oxygen and photosensitizer for alleviation of hypoxia tumor microenvironment and dramatically enhanced photodynamic therapy. Drug Deliv. 2018;25:585–99.
Zhao L, Li D, Zhang Y, Huang Q, Zhang Z, Chen C, Xu CF, Chu X, Zhang Y, Yang X. HSP70-Promoter-Driven CRISPR/Cas9 system activated by reactive oxygen species for multifaceted anticancer immune response and potentiated immunotherapy. ACS Nano. 2022;16:13821–33.
Ma Z, Zhang Y, Dai X, Zhang W, Foda MF, Zhang J, Zhao Y, Han H. Selective thrombosis of tumor for enhanced Hypoxia-Activated prodrug therapy. Adv Mater. 2021; 33.
Chen Z, Du Q, Guo W, Huang H, Li H, Zheng Y, Tan L, Fu C, Wu Q, Ren X, et al. Nanozymes-engineered metal–organic frameworks for enhanced microwave thermodynamic therapy in PDX of hepatic carcinoma. Chem Eng J. 2022;450:138092.
Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y, Du Y, Fang C, Tian J. A hepatocellular carcinoma targeting nanostrategy with Hypoxia-Ameliorating and photothermal abilities that, combined with immunotherapy, inhibits metastasis and recurrence. ACS Nano. 2020;14:12679–96.
Fan P, Zhang N, Candi E, Agostini M, Piacentini M, Centre TOR, Shi Y, Huang Y, Melino G. Alleviating hypoxia to improve cancer immunotherapy. Oncogene. 2023;42:3591–604.
Sufyan SA, van Devener B, Perez P, Nigra MM. Electronic tuning of gold nanoparticle active sites for reduction catalysis. ACS Appl Mater Interfaces. 2023;15:1210–8.
Lee HS, Yoo S-Y, Lee SM, Kang N-W, Kim SK, Song GY, Kim D-D, Lee J-Y. Hypoxia-alleviating hemoglobin nanoclusters for sensitizing chemo-photodynamic therapy of cervical cancer. Chem Eng J. 2023; 457.
Chen J, Liang X, Liu W, Gu W, Zhang B, Ji G. Mesoporous carbon Hollow spheres as a light weight microwave absorbing material showing modulating dielectric loss. Dalton Trans. 2019;48:10145–50.
Zhang W-J, Jin L-G, Wu S-L, Wang C-F, Zheng Y-F, Li Z-Y, Cui Z-D, Jiang H, Zhu S-L, Liu X-M. Microwave excited hyperthermy and catalysis of heterostructured Au/Cu–BTA for effective bacteria killing by accelerating charge separation. Rare Met. 2024;43:5186–201.
Wang J, Sun J, Hu W, Wang Y, Chou T, Zhang B, Zhang Q, Ren L, Wang H. A porous Au@Rh bimetallic Core-Shell nanostructure as an H2O2-Driven oxygenerator to alleviate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv Mater. 2020;32:e2001862.
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in cancer therapy: the bright side of the Moon. Exp Mol Med. 2020;52:192–203.
Wei W, Wang H, Ren C, Deng R, Qin Q, Ding L, Li P, Liu Y, Chang M, Chen Y, Zhou Y. Ultrasmall enzyodynamic PANoptosis Nano-Inducers for Ultrasound-Amplified hepatocellular carcinoma therapy and lung metastasis Inhibition. Adv Mater. 2024;36:e2409618.
Xu T, Ma Y, Yuan Q, Hu H, Hu X, Qian Z, Rolle JK, Gu Y, Li S. Enhanced ferroptosis by Oxygen-Boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano. 2020;14:3414–25.
Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71.
Chen Z, Guo W, Wu Q, Tan L, Ma T, Fu C, Yu J, Ren X, Wang J, Liang P, Meng X. Tumor reoxygenation for enhanced combination of radiation therapy and microwave thermal therapy using oxygen generation in situ by CuO nanosuperparticles under microwave irradiation. Theranostics. 2020;10:4659–75.
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–59.
Fan Z, Wu S, Deng H, Li G, Huang L, Liu H. Light-Triggered nanozymes remodel the tumor hypoxic and immunosuppressive microenvironment for Ferroptosis-Enhanced antitumor immunity. ACS Nano. 2024;18:12261–75.
Knocke S, Fleischmann-Mundt B, Saborowski M, Manns MP, Kuhnel F, Wirth TC, Woller N. Tailored tumor immunogenicity reveals regulation of CD4 and CD8 T cell responses against Cancer. Cell Rep. 2016;17:2234–46.
Zhao L, Tan L, Wu Q, Fu C, Ren X, Ren J, Wang Z, Zhang J, Meng X. A two-stage exacerbated hypoxia nanoengineering strategy induced amplifying activation of Tirapazamine for microwave hyperthermia-chemotherapy of breast cancer. J Colloid Interface Sci. 2024;659:178–90.
Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, Nedaeinia R, Haghjooy Javanmard S, Taherian M, Ahmadlou M, et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21:62.
Liu XL, Dong X, Yang SC, Lai X, Liu HJ, Gao Y, Feng HY, Zhu MH, Yuan Y, Lu Q, et al. Biomimetic liposomal nanoplatinum for targeted Cancer chemophototherapy. Adv Sci (Weinh). 2021;8:2003679.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (82302368), the Guangdong Basic and Applied Basic Research Foundation (2022A1515220167, 2021B1212040004) and Zhuhai Basic and Applied Basic Research Foundation (2320004002697), Guangzhou School (Hospital) Enterprise Joint Funding Project (Grant 2025A03J4320).
Author information
Authors and Affiliations
Contributions
Yitian Zhang and Bitao Li contributed equally to this work. Yitian Zhang: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data curation, Writing–original draft. Bitao Li: Conceptualization, Software, Formal analysis, investigation, Data curation, Validation, Resources, Writing-original draft. Jiawen He: Methodology, Formal analysis, investigation, Software, Validation, Writing-original draft. Ya Meng: Methodology, Formal analysis, Investigation, Software, Validation. Meixiao Zhan: Methodology, Investigation, Software, Validation, Writing-original draft. Cuixia Lu: Methodology, Investigation, Validation, Writing-original draft. Yong Li: Conceptualization, Supervision, Writing-review & editing, Funding acquisition. Feiyu Niu: Conceptualization, Supervision, Writing-review & editing, Funding acquisition. Liewei Wen: Conceptualization, Methodology, Supervision, Writing-review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
All the experiments were performed under protocols approved by the Animal Research Ethics Committee of Guangxi university (ethics approval number: GXU-2024-270).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Li, B., He, J. et al. Hemoglobin-loaded hollow mesoporous carbon-gold nanocomposites enhance microwave ablation through hypoxia relief. J Nanobiotechnol 23, 326 (2025). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12951-025-03387-x
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12951-025-03387-x