- Research
- Open access
- Published:
Insulin/PHMB-grafted sodium alginate hydrogels improve infected wound healing by antibacterial-prompted macrophage inflammatory regulation
Journal of Nanobiotechnology volume 23, Article number: 328 (2025)
Abstract
Background
Non-healing chronic wounds with high susceptibility to infection represent a critical challenge in modern healthcare. While growth factors play a pivotal role in regulating chronic wound repair, their therapeutic efficacy is compromised in infected microenvironments. Current wound dressings inadequately address the dual demands of sustained bioactive molecule delivery and robust antimicrobial activity.
Results
In this study, we developed a sodium alginate hydrogel (termed P-SA/Ins), which incorporated polyhexamethylene biguanide (PHMB) grafting and long-acting glargine insulin loading. P-SA/Ins exhibited the favorable physicochemical performance, biocompatibility and antibacterial efficacy against both Gram-negative and Gram-positive pathogens through inhibition of bacterial proliferation and biofilm formation. Glargine insulin was applied to prolonged insulin delivery. P-SA/Ins treatment attenuated S. aureus induced pro-inflammatory cytokine cascades in macrophages. The evaluation in vivo using a rat model with S. aureus infected wound demonstrated that P-SA/Ins significantly enhanced wound healing and optimized skin barrier through antimicrobial-mediated modulation of macrophage polarization and subsequent inflammatory cytokine profiling.
Conclusions
Our findings demonstrate that P-SA/Ins promotes wound healing and restores epidermal barrier integrity, indicating its potential as a therapeutic dressing for chronic wound healing, particularly in cases with infection risk.
Graphical abstract

Background
Due to the aging population and the rise of lifestyle diseases and their associated complications, non-healing wounds have become a “silent epidemic” [1]. These wounds affect impact the quality of life of nearly 2.5% of the total population in the United States [2]. Chronic unhealing wounds share several common features, including prolonged inflammation, persistent infections and impaired dermal and/or epidermal repair [3]. Optimal wound management strategies aim to reduce pain and hardship, as well as decreased morbidity, mortality and economic burden. Wound dressings are designed to provide a suitable microenvironment for healing. However, the need for frequent changes, adhesion to wound surfaces, and limited ability to provide a proper moist environment for wounds have made traditional wound dressings based on natural and synthetic fibers increasingly less desirable [4]. Advanced dressings, such as those made from hydrogels and hydrocolloids, which are characterized by better biocompatibility, degradability, and moisture retention, are the most commonly used dressings in clinical practice [5].
Alginate is one of the most practical polymer materials in the clinic due to its excellent hygroscopic capacity, gel-forming ability, pliability, and hemostatic properties. Alginate dressings can absorb exudate to double their own weight, maintaining a wet healing environment and accelerating wound healing [6]. D-gluconolactone can slowly hydrolyze and release protons in water, decreasing the pH of the solution. As a gelation-triggering agent, GDL induces the dissociation of CaCO₃ through pH reduction, which releases calcium ions. The released calcium ions crosslink with alginate to form a stable gel [7].
Unhealing wounds often exhibit persistent inflammation and an inadequate healing ability. Therefore, active molecules, such as growth factors or cytokines, are incorporated into hydrogels to accelerate the healing process [8]. However, growth factors, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) face limitations such as weak anti-inflammatory activity, short half-life, and potential oncogenicity [9,10,11,12]. In previous work, we explored the local application of low-dose insulin as a “growth factor-like” agent to enhance wound healing. Unlike conventional growth factors, insulin exerts pleiotropic effects for accelerating wound healing and improving healing quality through regulating inflammation, promoting angiogenesis, and basement membrane construction [13]. Clinical trials also demonstrated that local insulin promotes healing in acute and chronic wounds. Recently, a systematic review and network meta-analysis (NMA), with 23 reports (1240 patients), was processed to assess its safety and relative effectiveness. It identified local insulin statistically reduced wound area by 27%, increased healing rate by 23 mm/day, lowered Pressure Ulcer Scale for Healing (PUSH) scores by 2.7, shortened time to complete closure by 10 days, and achieved a 20-fold higher odds ratio for complete wound closure, with no significant adverse events [14]. However, the therapeutic application of biomolecules, such as insulin, for wound healing is challenging. Efforts are still being made to achieve its prolonged delivery in a localized and controlled manner, while ensuring the stability of the molecule [15, 16]. Furthermore, the application of insulin in infected wounds and the potential of long-acting insulin formulations to optimize delivery have been scarcely explored. In this study, we employed glargine insulin, a long-acting variant that is fully soluble in acidic environments (pH 4). The neutral environment precipitates it, resulting in small amounts of insulin release over a prolonged period.
Another important factor that prevents wounds from healing is bacterial infection, which compromises the efficacy of growth factors [17,18,19]. The topical application of antimicrobial agents is reported to be effective. In contrast to antibiotics, antiseptics are favored due to their broad antimicrobial spectrum and low risk of inducing bacterial resistance [20,21,22,23]. In this study, PHMB, a safe and commonly used antiseptic with a broad spectrum of effects against G+ and G− bacteria, fungi and even certain viruses, was selected as a polymer drug. On the market, PHMB has been used in various products, but no acquired microbial resistance has been reported [24].
Herein, we report the preparation, characterization and potential application of a biocomposite hydrogel formed by loading long-acting glargine insulin and PHMB onto sodium alginate (SA) and D-gluconolactone. In our work, 0.05% w/v PHMB was determined to be the optimal grafting concentration through comprehensive evaluation of physicochemical properties, antibacterial properties and biocompatibility. The pH change induced by GDL facilitated the assembly of glargine insulin into polymer, resulting in sustained release. In a full-layer skin infection wound rat model, P-SA/Ins accelerated wound healing and optimized the skin barrier via antibacterial-prompted macrophage inflammatory regulation (Scheme 1). These results indicate that P-SA/Ins is a promising candidate for infected wound dressings.
Materials and methods
Materials
Sodium alginate (SA) powder was purchased from Aladdin. Polyhexamethylene biguanide (PHMB), a 20% w/v aqueous solution (Cosmocil CQ), was obtained from Energy Chemical. GDL powder was obtained from Aladdin. Glargine insulin (300 IU/3 ml) was obtained from Sanofi-Aventis Deutschland GmbH. All other chemicals, NaCl and CaCO3, were purchased from local vendors. The CCK-8 and AO/EB staining kits were obtained from Sangon Biotech (Shanghai) Co., Ltd. E. coli (ATCC25922) and S. aureus (ATCC6538) were obtained from Nanjing Clinic Biological Technology Co., Ltd. The human umbilical vein endothelial cell (HUVEC) and human dermal fibroblast (HDF) cell lines were purchased from ASTRI Cell Resource Center (Shanghai).
Preparation of P-SA/Ins
Sodium alginate (0.9625 g) was continuously stirred until it was completely dissolved in 25 ml of deionized water at room temperature. Then, 0.4 g of CaCO3 powder was added. Moreover, a 1% w/v GDL solution was prepared. Next, 8 ml of GDL to get Sodium alginate hydrogel (SA). Then, glargine insulin (4.3 IU/ml final concentration) were mixed with SA/CaCO3 to get SA loading insulin (SA/Ins). Before gelation, every 10-ml mixture was cast in a 6-cm dish and allowed to stand at room temperature until cross-linking was complete. After cross-linking, the gel was cut with a 0.8-cm corneal trephine and abundantly rinsed with deionized water. Finally, the gel was immersed in PHMB for 10 min and washed with nongrafted PHMB to obtain P-SA/Ins.
Characterization of P-SA/Ins
The surface morphology and elemental composition of the gel were detected via field emission scanning electron microscopy (FE-SEM, Hitachi S-4800, Tokyo, Japan). FT-IR was measured in the range of 1000–4000 cm− 1 (Nicolet 5DX, MA, USA). The mechanical properties of the hydrogels were assessed via universal testing machines. With a 100 N sensor, the load speed was 5 mm/min. The swelling properties of the hydrogels were characterized. The W0hydrogel was placed in a test tube containing PBS to balance for a period of time. After complete hydration, the excess water on the gel surface was wiped with paper and weighed, and the weight was denoted as Wt. The swelling ratio was calculated as (Wt-W0)/W0*100%.
Kinetics of insulin release
-SA/Ins and P-SA/Ins (8-mm diameter) were separately added to 5 mL of PBS at 180 rpm and 37 °C. At set times, 1-ml aliquots of the release solution were collected, and fresh PBS was added to compensate for the volume removed. Insulin in the supernatants were analyzed using a human insulin ELISA kit (RayBiotech, Peachtree Corners, USA).
Antibacterial properties of P-SA/Ins in vitro
E. coli (ATCC25922, Nanjing Clinic Biological Technology Co., Ltd.) and S. aureus (ATCC6538, Nanjing Clinic Biological Technology Co., Ltd.) are representative strains of G- and G + bacteria. A disc diffusion assay was performed to assess the antibacterial properties of the materials. Moreover, the extraction solutions of each group and 100 µl of S. aureus bacterial mixture (OD = 0.2) were seeded and cultured in 96-well plates at 250 rpm and 37 °C. The OD value was determined after overnight culture. Moreover, scanning electron microscopy (SEM) was performed to assess bacterial morphology. Crystal violet staining was performed to assess biofilm formation in each group.
Cytotoxicity of P-SA/Ins in vitro
HUVECs and HDF cells were used for the cytotoxicity assay. AO/EB staining was performed to evaluate the toxicity of materials by assessing the apoptosis of HUVECs and/or HDFs after coculture with extraction solution of each group after 24 h. A scratch assay was used to assess the migration of HUVECs and HDFs. The viability of HUVECs and HDFs was detected with a CCK-8 assay.
Infected wound-healing assessment
Forty-eight SD rats were randomly assigned to 4 groups: Ctrl group were treated with Saline, SA group were treated with SA hydrogel, SA/Ins group were treated with SA/Ins hydrogel and P-SA/Ins group were treated with P-SA/Ins hydrogel. After anesthetization, the backs of the rats were shaved, and the hairs were removed once a day. S. aureus was grown as described above and diluted to 107 CFU/ml in PBS on the day of wounding. After the shaved area was cleaned and disinfected, four full-thickness dorsal wounds were established via 8-mm corneal trephines and scissors. The wound area was covered with 3 M Tegaderm (Cat. 1642 W). Sterile gauze and 100 µl of bacterial suspension were added to the wounds. The rats were administered the appropriate gels 48 h after bacterial inoculation. The Ctrl group was inoculated with sterile PBS. The dressing and gels were changed every other or two days, depending on the dressing conditions. The rats were maintained separately and supplied with unrestricted access to food and water. The wounds were photographed, and wound closure was measured at Days 0, 5, 9, and 14. The wound tissues and an additional 5 mm of the surrounding skin were collected.
Antibacterial properties in vivo
Under aseptic conditions, wound tissues were collected at 0, 5, 9, and 14 days after treatment. After being weighed, 1 ml of PBS was added to 100 mg of tissue for homogenization, and gradient dilution with PBS was performed. One hundred microliters from each dilution were inoculated on LB agar plates, and colony growth was observed after overnight culture. The bacterial colony number per gram (CFU/g) was calculated as colony number (CFU)× dilution factor/tissue weight (g).
Histology and Immunofluorescence
The harvested wound tissues were fixed in 10% polyformaldehyde solution. The fixed tissues were embedded in paraffin and sectioned. H&E and Masson’s trichrome staining were performed for histological and morphometric evaluation. Gram staining was used to detect bacteria. The multicolor IHC stain was performed according to the manufacturer’s recommendations. The primary and secondary antibodies used included mouse anti-rat CD68 (Bio-Rad, MCA341R), iNOS polyclonal antibody (Invitrogen, PA1-036), Arginase-1 rabbit mAb (Cell Signaling Technology, 93668), ZO-1 polyclonal antibody (Proteintech, 21773-1-AP), and Cytokeratin 14 (K14) polyclonal antibody (Proteintech, 10143-1-AP). Goat anti-rat IgG H&L (HRP) (Abcam, ab205720), goat anti-rabbit IgG H&L (HRP) (Abcam, ab205718), TSA assay kits from Runnerbio, Try-488 (Bry-try488), Try-cy3 (Bry-trycy3), and Try-cy5 (Bry-trycy5) were used. The quantification of the staining was performed via ImageJ software (National Institutes of Health, USA). At least six images were obtained for each group.
Luminex multiplex assay and enzyme-linked immunosorbent assay
To assess wound healing and inflammation-associated cytokines, Luminex multiplex assay rat kits (BIO-Plex, Pro Rat Cytokine 10plx) were used according to the manufacturer’s recommendations. The plates were read via a Luminex X-200. The conditioned media from primary macrophages derived from rat peritoneal fluid were extracted as previously described [25]. The macrophages were cocultured with S. aureus in the extraction solution of each group. Cultured cell supernatants were collected for subsequent experiments. The wound tissue was harvested at 5, 9, and 14 days after injury and stored at − 80 °C. After homogenization, the lysates were incubated on ice for 30 min before centrifugation at 12,000 rpm for 15 min. The supernatants were collected and stored at -80 °C. Insulin levels were detected in the wound tissue 9 days after injury via an insulin ELISA kit.IL-1β, IL-10, IL-13 and TNF-α levels in the wound tissue at 5 and 14 days after injury were measured via the Luminex multiplex assay.
Statistical analysis
All the data were obtained from at least three independent experiments and are presented as the means ± SDs. Data analysis was performed via GraphPad Prism. The significant differences were determined via one-way ANOVA (Dunnett’s post hoc test) and evaluated via a standard two-tailed Student’s t test. Differences were considered significant when *p < 0.05, **p < 0.01 or ***p < 0.001.
Results
Characterization of P-SA/Ins
When the GDL was added, the mixture with glargine insulin was crosslinked slowly and evenly for approximately 30 min ~ 1 h at RT. And Macroscopic images of the hydrogels were shown in Fig. 1A. SEM revealed that alginate forms a highly crosslinked network structure after crosslinking with calcium ions, as shown in Fig. 1B. After insulin loading, the overall structure of the hydrogel did not change significantly, indicating that insulin loading did not damage the structure of the hydrogel. After the grafting of PHMB was completed, a PHMB membrane formed on its surface, indicating successful PHMB loading. The successful grafting of PHMB was verified by FI-TR spectra (Fig. 1C). In the spectrogram of the P-SA/Ins, the characteristic infrared absorption peaks of PHMB appeared at 2150 cm− 1 and 2900 cm− 1, representing the absorption peaks of the C = N bond and C-O bond, respectively. In addition, a vibrational absorption peak appeared at 1400 cm− 1, indicating amide bond formation. Figure 2D and Table S1 show that P-SA/Ins has a swelling rate of 200%, which plays an important role in absorbing wound tissue exudate. Glargine insulin has the ability to stimulate stable and prolonged insulin release for 6–24 h in a neutral environment [26]. In this program, the acidic environment created by the GDL maintained the soluble state of glargine insulin. With the reaction of the system and cross-linking completion, the acidity was neutralized, and insulin was precipitated to achieve stable and sustainable release for at least 96 h. The cumulative release of insulin from SA/Ins and P-SA/Ins in PBS was approximately 689.8 ± 25.5 mIU and 664.2 ± 28.2 mIU within 7 days (Fig. 1E).
Characterization of P-SA/Ins. A: Schematic and general view of P-SA/Ins. B: SEM images of each group. The scales bars: 500 μm and 100 μm. C: FI-TR spectra of gels of each group. D: The swelling ratio of each group in 37℃ PBS within 48 h. E: The cumulative released amount of insulin from SA/Ins and P-SA/Ins incubated in 37℃ PBS
Biocompatibility of P-SA/Ins
AO/EB staining, CCK-8 assays and scratch assays were subsequently performed to evaluate the biocompatibility of the P-SA/Ins by observing the viability, proliferation and migration of HUVECs and HDFs. The viability of HUVECs and HDFs was assessed via an AO/EB assay. The cells were cultured with an extraction solution of endothelial cell medium or DMEM from SA, SA/Ins or P-SA/Ins (0.05%, w/v)). Figure 2A-B shows that the number of dead cells (stained orange by EB) in each group was similar to that in the control group for 24 h, indicating that P-SA/Ins had barely toxicity. Cell proliferation under P-SA/Ins was detected via a CCK-8 assay. Compared with other groups, the growth of HDF (Fig. 2D) and HUVEC (Fig. 2E) from the SA/Ins and P-SA/Ins groups was significantly greater at 3 and 5 days. PHMB grafting had no effect on insulin-induced HDF or HUVEC growth. For wound healing assay, the migration rates of the HDFs and HUVECs in the SA/Ins group reached 89% and 93%, whereas those in the P-SA/Ins group reached 88% and 90%, which were significantly greater than those in the control group (65% for HDFs and 78% for HUVECs) and the SA group (68% for HDFs and 76% for HUVECs) (Fig. 2F-I). Based on these results, P-SA/Ins was able to facilitate fibroblast and endothelial cell proliferation and migration, with barely cytotoxicity.
Biocompatibility of P-SA/Ins. A-C: Representative images and statistical data of Cell Toxicity assay of HDF and HUVEC cell for 24 h. (ns: p > 0.05, scales bars: 0.5 mm). D-E: The statistical data of cell growth of HDF (D) and HUVEC (E). (** p ≤ 0.01, * p ≤ 0.05) F-G: Wound healing assay and statistical data of HDF(F-G) and HUVEC (H-I). (Scale bar: 1 mm. *** p ≤ 0.001, * p ≤ 0.05)
Antibacterial ability of P-SA/Ins
E. coli and S. aureus were used as representative strains of G- and G + bacteria for antibacterial ability. Studies have reported that 0.019–2% PHMB is applied to kill bacterial [27]. The concentration of PHMB grafted on the sodium alginate hydrogel was fully evaluated. An antibacterial ability of hydrogels with PHMB concentrations on 10− 5%, 10− 4%,10− 3%, 10− 2%, 0.05%, 0.1%, 0.2%, 0.5%, 1%, 2%, and 5% w/v was conducted. A disk diffusion assay was performed to evaluate antibacterial activity by measuring the zone of inhibition (ZOI), with penicillin‒streptomycin used as a positive control. As shown in Figure S1A-B, 10− 5%, 10− 4%,10− 3% PHMB grafted didn’t show the zone of inhibition, indicating that they had no bactericidal effect. When the PHMB concentration was greater than 0.2%, the antibacterial effect did not increase with increasing PHMB concentration. The diameters of the ZOIs for E. coli were approximately 12, 15, 20 mm and more than 22 mm for 10− 2%, 0.05%, 0.1%, 0.2% PHMB-grafted. For S. aureus, approximately 10 mm, 13 mm, 15 mm and 16 mm for 10− 2%, 0.05%, 0.1%, and 0.2% PHMB-grafted, respectively. To further optimize the concentration of PHMB, the viability of HDFs cultured with extraction solution from the 10− 2%, 0.05%, 0.1%, and 0.2% P-SA/Ins was assessed by an AO/EB assay for 24 h. As shown in Figure S2, the number of early apoptotic or dead cells in the 10− 2% and 0.05% groups was close to that in the control group, indicating their good cytocompatibility. When the concentration of PHMB was greater than 0.05% w/v, the number of dead cells drastically increased and reached approximately 15%, indicating an adverse effect on cell viability and cytotoxicity. Moreover, the hydrogels immersed in PBS for 3 and 5 days still exhibited strong antibacterial effects on both S. aureus and E. coli, with 12–13 mm inhibition diameters (Fig. 3A-D). Thus, 0.05% w/v PHMB was considered the optimal concentration for P-SA/Ins.
The antibacterial effect of P-SA/Ins (0.05%, w/v) was also assessed via an OD value counting assay. Compared with other groups, the leaching culture medium of the P-SA/Ins substantially reduced the bacterial density of S. aureus and E. coli, as shown by the bacterial optical density (Fig. 3B). A crystal violet assay for biofilm biomass indicated that P-SA/Ins was more effective in alleviating the formation of S. aureus biofilms (Fig. 3C). Visualization of the bacterial biofilms via SEM revealed a wide spectrum of morphological differences (Fig. 3E). Unlike the good condition and aggregation of S. aureus cells in other groups (Red arrow), scattered and crimpled cells (Yellow arrow) were observed after exposure to the leaching culture medium of P-SA/Ins. Taken together, these results demonstrated that P-SA/Ins effectively inhibited bacterial activity and biofilm formation in both G- and G + bacteria.
Regulation of macrophage inflammation by P-SA/Ins
Macrophages are important inflammatory cells involved in wound regulation [28]. To evaluate the effect of P-SA/Ins on the inflammatory response of macrophages in the presence of infection, macrophages were cocultured with S. aureus in the leaching culture medium of each group for 24 h. The cell culture supernatant was collected and analyzed via a Luminex multiplex assay to detect typical M1- and M2-related cytokines (Fig. 3F). A heatmap revealed that the P-SA/Ins group had distinguishable protein profiles from those of the other three groups (Fig. 3G). The levels of Interleukin-1β (IL-1β), Interleukin-1α (IL-1α), Tumor necrosis factor-α (TNF-α) and Interleukin-6 (IL-6) in the P-SA/Ins group were significantly lower than those in the Ctrl group. There were significant differences in the levels of IL-1β, TNF-α, IL-1α and Interferon–γ (INF-γ) (p < 0.05) (Fig. 3H-K). In addition, the typical anti-inflammatory cytokine Interleukin-13 (IL-13) increased (Fig. 3L). These results suggested that insulin alone could not effectively inhibit the inflammatory response of macrophages induced by bacteria. Suppresses bacterial activity effectively controls the release of inflammatory cytokines, and the levels of anti-inflammatory factors increase to a certain extent.
Antibacterial and inflammation regulation of P-SA/Ins in vitro. A: Schematic of antibacterial assay of P-SA/Ins. B: The statistical data of OD value counting assay of S. aureus. (***p ≤ 0.001, n = 12). C: The statistical data of crystal violet assay for S. aureus biofilm formation (*** p ≤ 0.001, n = 9). D: The statistical data of the diameters of inhibition zones for S. aureus and E. coli. E: Representative SEM images showed S. aureus. The scales bars: 1 μm. Red arrow: the good condition and aggregation of S. aureus; Yellow arrow: scattered and crimpled S. aureus. F: Schematic of antibacterial and inflammation regulation of P-SA/Ins (scar bar: 1 μm). G: Heatmap of inflammatory cytokines of leaching culture medium cultured with macrophage and S. aureus. H-L: The statistical data of IL-1β, TNF-α, IL-1α, IFN-γ, IL-13 in leaching culture medium of cultured with macrophage and S. aureus. (ns: p > 0.05, ** p ≤ 0.01, * p ≤ 0.05.)
Effects of P-SA/Ins on wound healing
To verify the effects of P-SA/Ins treatment on the healing of infected wounds. A rat model of wound infection was established [29]. P-SA/Ins treatment was given, the healing process was recorded, and the wound samples were collected as shown in Fig. 4A. Photographs of healing wounds revealed that P-SA/Ins treatment efficiently accelerated wound healing at 5, 9, and 14 days (Fig. 4B-C). On Day 5, the percentage of the nonhealing wound area was 53.0 ± 3.2% in the P-SA/Ins group, and those in the Ctrl, SA, and SA/Ins groups were 71.1 ± 2.4%, 67.2 ± 5.9%, and 60.3 ± 7.8%, respectively. On Day 9, the percentage of the nonhealing wound area in the P-SA/Ins group (9.3 ± 1.2%) was significantly less than that in the Ctrl (32.9 ± 2.7%), SA (26.5 ± 2.5%), and SA/Ins (16 ± 2.9%) groups. On Day 14, the wounds in the P-SA/Ins group were healed already, whereas the nonhealing wound area percentages in the Ctrl, SA, and SA/Ins groups were 12.3 ± 7.5%, 7.2 ± 1.7%, and 3.4 ± 1.5%, respectively. H&E staining revealed that the P-SA/Ins substantially accelerated the wound healing rate. After 14 days of treatment, the wounds completely closed, with rapid re-epithelialization (Fig. 4D-F). The general view of the regenerated area and the infiltration of inflammatory cells were shown in Figure S3A-B. Moreover, the P-SA/Ins increased collagen deposition (Fig. 4E, G). These data suggest that P-SA/Ins promote healing of infected wounds.
Effect of wound healing of P-SA/Ins. A: The establishment of rat model with infected full thickness excision dorsal wounds. B: Photographs of healing wounds of rats. C: Quantitative analysis of percentage of wound area. (SA vs. SA/Ins., #P < 0.05; SA vs. P-SA/Ins, ***P < 0.01, **P < 0.01, *P < 0.05, n = 6). D-E: H&E (D) and Masson (E) staining of rat wounds on 14 days after treatment (Scale bar: 1 mm). F: The statistical data of the length of epidermal tongue (ns: p > 0.05, ** p ≤ 0.01, * p ≤ 0.05, n = 6). G: The statistical data of the collagen content (ns: p > 0.05, **** p ≤ 0.0001, *** p ≤ 0.001, n = 6)
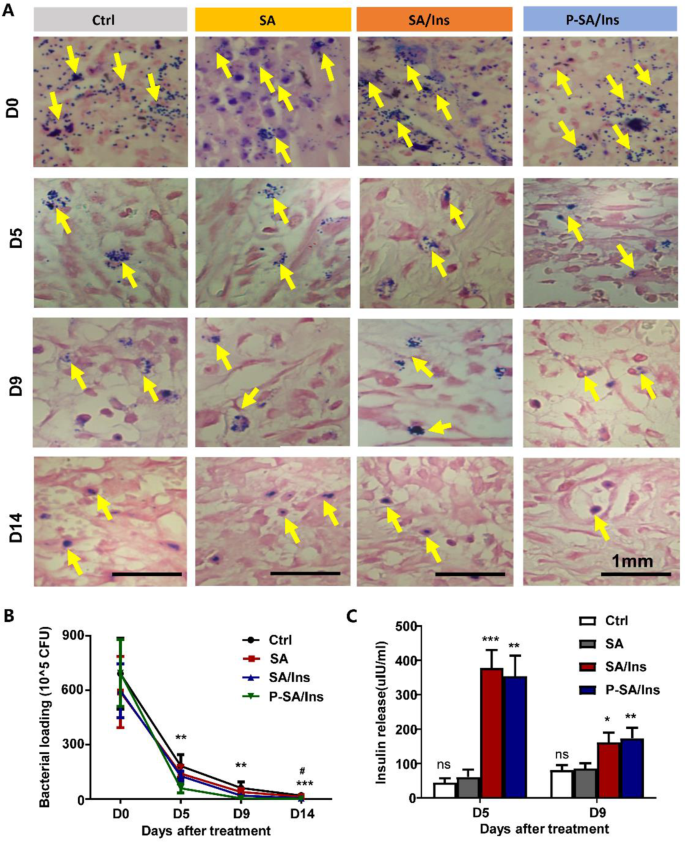
Antibacterial properties of P-SA/Ins in vivo
At 0, 5, 9, and 14 days after treatment, the wound tissues were harvested and processed for bacterial analysis. Gram staining revealed that the bacteria (Yellow arrow) in the tissues under P-SA/Ins treatment was significantly lower than that in the other three groups at 0, 5, 9, and 14 days (Fig. 5A). The bacteria in the wound tissues decreased rapidly in each group. However, the P-SA/Ins treatment had significantly fewer bacteria (Fig. 5B). In addition, SA/Ins treatment also reduced the number of bacteria on Day 14, which may partially attribute to immunomodulatory effect of insulin [30].
Insulin concentrations in wounds
To evaluate the insulin release in the wound, the wound tissues were harvested at 5 and 9 days. After homogenization, the insulin level of the wound extract was detected via ELISA. Figure 5C shows that the insulin level in the wound tissue from the SA/Ins (378.3 ± 51.8 mIU) and P-SA/Ins (354.3 ± 59.3 mIU) groups was significantly greater than that in the Ctrl (44.6 ± 12.4 mIU) and SA (61.2 ± 20.9 mIU) treatment groups. A similar trend was also observed on Day 9 (SA/Ins: 161.8 ± 28.4 mIU; P-SA/Ins: 173.5 ± 30.4 mIU; Ctrl: 80.9 ± 14.2 mIU; SA: 85.9 ± 15.1 mIU), suggesting that insulin was released effectively into the wounds.
Antibacterial effect of P-SA/Ins in wound healing. A: The representative images of Gram stain to detect S. aureus (Yellow arrow) in wound tissue on 14 days after treatment (Scale bar: 1 mm). B: The amount of S. aureus in wound tissue after treatment for 0, 5, 9, and 14 days. Ctrl vs. SA/Ins, # P < 0.05. Ctrl vs. P-SA/Ins, ***P < 0.01, **P < 0.01; n = 6). C: The insulin concentration in wound. (***P < 0.01, **P < 0.01, *P < 0.05, n = 6)
Regulation of infected wound inflammation by P-SA/Ins
To examine the inflammatory state in the wound, typical inflammatory factors and M1/M2 markers were detected in the wound. Multicolor fluorescence was used to determine the proportion of iNOS-marked M1 macrophages and Arg-1-marked M2 macrophages in the wound on Day 9 after treatment (Fig. 6A). Compared with those in the Ctrl (39.67\(\:\pm\:\)10.08%) and SA (26.33\(\:\pm\:\)4.19%) groups, the numbers of macrophages in wounds in the SA/Ins (20.33\(\:\pm\:\)0.94%) were decreased. However, the proportion of macrophages was further reduced (14.67\(\:\pm\:\)3.09%), with Arg-1-marked macrophages (55\(\:\pm\:\)0.09%) being the predominant phenotype in the P-SA/Ins group (Figure S4). Collectively, these observations indicate that P-SA/Ins decreased macrophage infiltration and promotes the M2 phenotype in wounds. Furthermore, high amounts of the inflammatory and anti-inflammatory factors IL-13, IL-10, IL-1β, and TNF-α were observed in the Ctrl, SA, SA/Ins groups at Days 5 and 9 after treatment. P-SA/Ins treatment substantially increased the level of IL-13, blunted the levels of Interleukin-10 (IL-10) and the inflammatory factors IL-1β and TNF-α, indicating that P-SA/Ins markedly alleviated inflammation in the wounds (Fig. 6B-E).
Optimized healing of infected wounds by P-SA/Ins
Since infected wounds are prone to blisters and repeated ulcers due to poor epidermal and dermal connections, we further explored the effects of the P-SA/Ins on the healing quality of infected wounds on Day 14. Compared with the other 3 groups, the epidermis (Black dash line) was closely connected to the dermis with extensive skin nails (Red arrow), as shown by Masson’s staining under P-SA/Ins treatment (Fig. 7A, C). Immunofluorescence of keratin 14 (White arrow) suggested excellent continuity of the basement membrane (White dashed line) in Fig. 7B. Tight junction ZO-1 (White arrow) staining revealed compact junctions between keratinocytes, indicating better integrity and stability of the skin barrier (Fig. 7C-E). Thus, we determined that the P-SA/Ins optimized the healing of infected wounds via rapid re-epithelialization and strengthen the skin barrier and basement membrane.
Optimized healing of infected wounds by P-SA/Ins. A: The skin nails in zoom-in of Masson staining. Scale bar: 250 μm. Black dashed line: the epidermis. Red arrow: basement membrane and skin nails. B: Immunofluorescent staining showed K14, ZO-1in rat wounds on 14 days after treatment. Scale bar: 50 μm. White dashed line: basement membrane. White arrow: Expression and distribution of K14, ZO-1 C: The statistical data of skin nails in rat wounds. (ns: p > 0.05, **P < 0.01, n = 5) D: The statistical data of K14 positive rate cell in rat wounds. (ns: p > 0.05, **P < 0.01, n = 5). E: The statistical data of ZO-1 positive rate cell in rat wounds. (ns: p > 0.05, *P < 0.05, n = 5)
Discussion
Wound healing involves overlapping and integrated phases of hemostasis, inflammation, proliferation and remodeling [31]. Among the various reasons, infection is one of the most frequent causes and potentially delays or, in some cases, causes the wound to not heal. Furthermore, wound infection can also lead to complications, such as osteomyelitis, septicemia, and even death [32]. Commercially available wound dressings, such as Aquacel®, Comfeel®, DuoDerm®, Granuflex®, and Tegasorb® wound dressing materials, are not suitable for infected wounds. In addition, they are highly priced and carry the risk of infection and antigenicity [33]. Therefore, the presence of bacteria is one of the greatest challenges in the wound healing process. Currently, the treatment of infected wounds is considered an unmet clinical need.
Polysaccharide-based hydrogels display appropriate biomaterial properties, such as biodegradability, biocompatibility, hygroscopicity, and cost-effectiveness. Alginate, which is characterized by high hygroscopicity, glue-forming and hemostatic properties, and soft and easy folding, is suitable for many advanced clinical and biomedical applications. Like other wound dressings, commercially available alginate wound dressing materials, such as Nu-derm®, Algisite M®, and Melgisorb®, are not suitable for infected wound applications because of their insufficient antimicrobial activity. Moreover, most bioactive molecules associated with inadequate healing have potential oncogenicity and weak inflammation regulation [12]. Insulin has been shown to act as a “growth-like factor” by accelerating wound healing and improving healing quality [13, 34]. However, the disadvantage of insulin is its short half-life.
In this study, we developed a sodium alginate hydrogel loaded with long-acting insulin and PHMB for the treatment of infected wounds. PHMB is a cationic antimicrobial. Its positive charge easily interacts with anionic groups [35]. Thus, PHMB interacts with negative components of the microorganism cell wall, resulting in disruption and death of the bacteria. Moreover, PHMB can penetrate and bind to the DNA of several bacterial species, condense their chromosomes and prevent cell division [36]. Studies have reported that 0.019–2% PHMB were applied [27, 37]. Through comprehensive evaluation, the optimal grafting concentration of PHMB, a safe broad-spectrum antiseptic, on sodium alginate was determined to be 0.05% w/v. The P-SA/Ins (0.05% w/v) provided persistent antimicrobial activity and effective wound infection prevention. Moreover, the survival, proliferation and migration of endothelial cells and fibroblasts ensure good biocompatibility of P-SA/Ins. Furthermore, GDL is used to control pH and realize the loading of long-acting glargine insulin, resulting in low-dose sustained release of insulin.
The inflammatory response, as the first stage of several overlapping wound healing phases, has been reported to play essential roles in the orchestration of transitions among the three healing phases [38]. Macrophages constitute important immunomodulatory cells and regulate wound healing through multiple mechanisms, such as phagocytosis, inflammation initiation and resolution, and growth factor secretion, to promote cell proliferation and tissue recovery in wounds [39]. Their phenotype readily changes according to spatiotemporal cues during repairment. Unhealing wounds are closely associated with sustained infiltration and impaired phenotypic transition from proinflammatory to anti-inflammatory phenotypes [40].
Therefore, we examined the effects of P-SA/Ins on the secretion of inflammatory cytokines by macrophages under S. aureus stimulation in vitro. These results suggested that P-SA/Ins dramatically reduced the release of IL-1β, IL-1α, TNF-α and IL-6 from macrophages induced by S. aureus. The typical anti-inflammatory cytokines IL-13 and IL-4 were increased (Fig. 4I). In addition, insulin is generally believed to regulate the inflammatory response of macrophages [13], but our results revealed that the regulatory effect of insulin on inflammation was not significant during infection. Interestingly, a high-affinity insulin-binding protein secreted by S. aureus was reported to mediate insulin resistance in a mouse model of infection [41]. Thus, we propose that P-SA/Ins regulates inflammation in macrophages by controlling bacteria and infection. Similarly, in vivo, P-SA/Ins effectively eliminated bacteria from wounds. Compared with the robust inflammatory factors IL-1β and TNF-α and a large number of M1-dominant macrophages in infected wounds, P-SA/Ins not only effectively blunted the levels of macrophage proinflammatory factors but also promoted the M2 phenotype of macrophages and macrophage regression.
Another challenge is the occurrence of blisters and the repeated ulceration of infected wounds that have completed epithelization, especially chronic infected wounds. As the largest organ of the human body, the skin comprises three distinct layers: the epidermis, dermis, and hypodermis. The epidermal and dermal layers are separated from each other by a basement membrane termed the dermo-epidermal junction. The complex dermo-epidermal junction structure comprises keratinocytes with K5 and K14 expression, ECM components, the basal lamina, filaments, and anchoring fibrils. In addition, the better integrity and stability of the skin barrier are closely related to the formation of cell‒cell junctions [42]. It is reported that S. aureus damages lipid and corneocyte barriers by producing Sal2/Geh lipase and potent proteases, respectively. The scalded skin toxin, found in a subset of S. aureus isolates, is a protease that disintegrates stratum corneum integrity [43]. Cytokines are responsible for the control of cellular communication. Cytokine signaling can affect the physiology of keratinocytes and the quality of the skin barrier [44]. IL-1β stimulation for 96 h downregulated the expression of thigh junction proteins, including occludin, claudin-1 and ZO-1 [45]. The serum levels of TNF-α in suction skin blister fluids are correlated with the severity of psoriasis. In psoriasis patients, TNF-α prevents proper barrier formation by inhibiting the expression of FLG and LOR, resulting in weakening of the skin barrier [46].The detection of K14 and ZO-1, which connect tight junctions, demonstrated that P-SA/Ins optimized the healing qualities of infected wounds by enhancing the continuity of the basement membrane and compact junctions between keratinocytes. This may be due to the remarkable reduction in the S. aureus load and the regulation of the inflammatory environment in the wound by P-SA/Ins.
Conclusion
In this study, we developed a sodium alginate hydrogel loaded with long-acting insulin and grafted with PHMB and demonstrated its therapeutic effects on infected wounds in vitro and in vivo. The P-SA/Ins composite provided persistent antimicrobial activity and effective wound infection prevention. GDL is applied to and realize the loading of insulin and sustained release of long-acting insulin by pH control. Based on the results of animal studies of infected wounds in rats, we further demonstrated that P-SA/Ins was a practical therapeutic solution for the treatment of infected wounds by antibacterial-prompted macrophage inflammatory regulation. Given the aforementioned effectiveness, the maturity of the raw material and preparation process and the known merits of hydrogels, they have great potential and application prospects for infected wound healing.
Data availability
No datasets were generated or analysed during the current study.
References
Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019;27(1):114–25. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/wrr.12683.
Sen CK. Human wound and its burden: updated 2020 compendium of estimates. Adv Wound Care (New Rochelle). 2021;10(5):281–92. https://doiorg.publicaciones.saludcastillayleon.es/10.1089/wound.2021.0026.
Liu W, Zu L, Wang S, Li J, Fei X, Geng M, et al. Tailored biomedical materials for wound healing. Burns Trauma. 2023;11:tkad040. https://doiorg.publicaciones.saludcastillayleon.es/10.1093/burnst/tkad040.
Hawthorne B, Simmons JK, Stuart B, Tung R, Zamierowski DS, Mellott AJ. Enhancing wound healing dressing development through interdisciplinary collaboration. J Biomed Mater Res B Appl Biomater. 2021;109(12):1967–85. https://doiorg.publicaciones.saludcastillayleon.es/10.1002/jbm.b.34861.
’t Op RC, Walboomers XF, Jansen JA, Wagener F. Design considerations for hydrogel wound dressings: strategic and molecular advances. Tissue Eng Part B Rev. 2020;26(3):230–48. https://doiorg.publicaciones.saludcastillayleon.es/10.1089/ten.TEB.2019.0281.
Shen S, Chen X, Shen Z, Chen H. Marine polysaccharides for wound dressings application: an overview. Pharmaceutics. 2021;13(10). https://doiorg.publicaciones.saludcastillayleon.es/10.3390/pharmaceutics13101666.
Komoto D, Furuike T, Tamura H. Preparation of polyelectrolyte complex gel of sodium alginate with Chitosan using basic solution of Chitosan. Int J Biol Macromol. 2019;126:54–9. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.ijbiomac.2018.12.195.
Weng TT, Cai CH, Han CM, Wang XG. [Research advances on biomaterials for the delivery of growth factors to regulate wound repair]. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022;38(7):691–6. https://doiorg.publicaciones.saludcastillayleon.es/10.3760/cma.j.cn501225-20220430-00166.
Koupai AA, Varshosaz J, Dobakhti F, Shekarchizadeh F, Al-Musawi MH, Kamil MM, et al. Vanillin and IGF1-loaded dual-layer multifunctional wound dressing with micro-nanofibrous structure for full-thickness wound healing acceleration. Int J Pharm. 2025;671:125231. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.ijpharm.2025.125231.
Shahriari-Khalaji M, Sattar M, Wei H, Al-Musawi MH, Ibrahim Yahiya Y, Hasan Torki S, et al. Physicochemically Cross-linked injectable hydrogel: an adhesive skin substitute for burned wound therapy. ACS Appl Bio Mater. 2025;8(2):1292–306. https://doiorg.publicaciones.saludcastillayleon.es/10.1021/acsabm.4c01592.
Tavakoli M, Al-Musawi MH, Kalali A, Shekarchizadeh A, Kaviani Y, Mansouri A, et al. Platelet rich fibrin and simvastatin-loaded pectin-based 3D printed-electrospun bilayer scaffold for skin tissue regeneration. Int J Biol Macromol. 2024;265(Pt 1):130954 https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.ijbiomac.2024.130954.
Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20(2):207–17. https://doiorg.publicaciones.saludcastillayleon.es/10.1007/s11154-019-09492-1.
Yu T, Gao M, Yang P, Liu D, Wang D, Song F, et al. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J Cell Physiol. 2019;234(4):4217–31. https://doiorg.publicaciones.saludcastillayleon.es/10.1002/jcp.27185.
Ramirez-GarciaLuna JL, Rangel-Berridi K, Bergeron A, Kolosovas-Machuca ES, Wang SC, Berry GK, et al. Local insulin improves wound healing: A systematic review and bayesian network Meta-Analysis. Plast Reconstr Surg. 2023;152(6):e1114–30. https://doiorg.publicaciones.saludcastillayleon.es/10.1097/prs.0000000000010432.
Aguilar-Vázquez R, Romero-Montero A, Del Prado-Audelo ML, Cariño-Calvo L, González-Del Carmen M, Vizcaíno-Dorado PA, et al. Biopolymeric insulin membranes for antimicrobial, antioxidant, and wound healing applications. Pharmaceutics. 2024;16(8). https://doiorg.publicaciones.saludcastillayleon.es/10.3390/pharmaceutics16081012.
Walther M, Vestweber PK, Kühn S, Rieger U, Schäfer J, Münch C, et al. Bioactive Insulin-Loaded electrospun wound dressings for localized drug delivery and stimulation of protein expression associated with wound healing. Mol Pharm. 2023;20(1):241–54. https://doiorg.publicaciones.saludcastillayleon.es/10.1021/acs.molpharmaceut.2c00610.
Firuzeh M, Labbaf S, Enayati MH, Dinari M, Mirhaj M. Enhanced wound healing with a bilayered multifunctional quaternized chitosan-dextran-curcumin construct. Carbohydr Polym. 2025;352:123195. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.carbpol.2024.123195.
Metcalf DG, Bowler PG. Biofilm delays wound healing: A review of the evidence. Burns Trauma. 2013;1(1):5–12. https://doiorg.publicaciones.saludcastillayleon.es/10.4103/2321-3868.113329.
Schiffer D, Blokhuis-Arkes M, van der Palen J, Sigl E, Heinzle A, Guebitz GM. Assessment of infection in chronic wounds based on the activities of Elastase, lysozyme and myeloperoxidase. Br J Dermatol. 2015;173(6):1529–31. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/bjd.13896.
Almajidi YQ, Muslim RK, Issa AA, Al-Musawi MH, Shahriari-Khalaji M, Mirhaj M. Three-dimensional printed polyelectrolyte construct containing mupirocin-loaded quaternized Chitosan nanoparticles for skin repair. Int J Biol Macromol. 2024;280(Pt 4):136214 https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.ijbiomac.2024.136214.
Mirhaj M, Varshosaz J, Labbaf S, Emadi R, Marcus Seifalian A, Sharifianjazi F. An antibacterial Multi-Layered scaffold fabricated by 3D printing and electrospinning methodologies for skin tissue regeneration. Int J Pharm. 2023;645:123357. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.ijpharm.2023.123357.
Mirhaj M, Varshosaz J, Labbaf S, Emadi R, Seifalian AM, Sharifianjazi F, et al. Mupirocin loaded core-shell pluronic-pectin-keratin nanofibers improve human keratinocytes behavior, angiogenic activity and wound healing. Int J Biol Macromol. 2023;253(Pt 2):126700 https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.ijbiomac.2023.126700.
Ramasamy S, Muthusamy S, Nagarajan S, Nath AV, Savarimuthu JS, Jayaprakash J, et al. Fabrication of collagen with polyhexamethylene Biguanide: A potential scaffold for infected wounds. J Biomed Mater Res B Appl Biomater. 2022;110(3):535–46. https://doiorg.publicaciones.saludcastillayleon.es/10.1002/jbm.b.34933.
Kamaruzzaman NF, Firdessa R, Good L. Bactericidal effects of polyhexamethylene Biguanide against intracellular Staphylococcus aureus EMRSA-15 and USA 300. J Antimicrob Chemother. 2016;71(5):1252–9. https://doiorg.publicaciones.saludcastillayleon.es/10.1093/jac/dkv474.
Liu D, Yang P, Gao M, Yu T, Shi Y, Zhang M, et al. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci (Lond). 2019;133(4):565–82. https://doiorg.publicaciones.saludcastillayleon.es/10.1042/cs20180600.
Vargas-Uricoechea H. Efficacy and safety of insulin glargine 300 U/mL versus 100 U/mL in diabetes mellitus: A comprehensive review of the literature. J Diabetes Res. 2018;2018:2052101. https://doiorg.publicaciones.saludcastillayleon.es/10.1155/2018/2052101.
Jin J, Chen ZL, Xiang Y, Tang T, Zhou H, Hong XD, et al. Development of a PHMB hydrogel-modified wound scaffold dressing with antibacterial activity. Wound Repair Regen. 2020;28(4):480–92. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/wrr.12813.
He WF, Yan LF. [The regulatory role and related mechanisms of macrophages in wound healing]. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023;39(2):106–13. https://doiorg.publicaciones.saludcastillayleon.es/10.3760/cma.j.cn501225-20230110-00010.
Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. Animal models of external traumatic wound infections. Virulence. 2011;2(4):296–315. https://doiorg.publicaciones.saludcastillayleon.es/10.4161/viru.2.4.16840.
Chen X, Liu Y, Zhang X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair Regen. 2012;20(3):425–34. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/j.1524-475X.2012.00792.x.
Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. https://doiorg.publicaciones.saludcastillayleon.es/10.1098/rsob.200223.
Malone M, Schultz G. Challenges in the diagnosis and management of wound infection. Br J Dermatol. 2022;187(2):159–66. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/bjd.21612.
Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application:a mini review. Carbohydr Polym. 2020;236:116025. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.carbpol.2020.116025.
Liu Y, Petreaca M, Yao M, Martins-Green M. Cell and molecular mechanisms of keratinocyte function stimulated by insulin during wound healing. BMC Cell Biol. 2009;10:1. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/1471-2121-10-1.
Napavichayanun S, Amornsudthiwat P, Pienpinijtham P, Aramwit P. Interaction and effectiveness of antimicrobials along with healing-promoting agents in a novel biocellulose wound dressing. Mater Sci Eng C Mater Biol Appl. 2015;55:95–104. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.msec.2015.05.026.
Chindera K, Mahato M, Sharma AK, Horsley H, Kloc-Muniak K, Kamaruzzaman NF, et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci Rep. 2016;6:23121. https://doiorg.publicaciones.saludcastillayleon.es/10.1038/srep23121.
de Mattos IB, Holzer JCJ, Tuca AC, Groeber-Becker F, Funk M, Popp D, et al. Uptake of PHMB in a bacterial nanocellulose-based wound dressing: A feasible clinical procedure. Burns. 2019;45(4):898–904. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.burns.2018.10.023.
Li M, Hou Q, Zhong L, Zhao Y, Fu X. Macrophage related chronic inflammation in Non-Healing wounds. Front Immunol. 2021;12:681710. https://doiorg.publicaciones.saludcastillayleon.es/10.3389/fimmu.2021.681710.
Hassanshahi A, Moradzad M, Ghalamkari S, Fadaei M, Cowin AJ, Hassanshahi M. Macrophage-Mediated inflammation in skin wound healing. Cells. 2022;11(19). https://doiorg.publicaciones.saludcastillayleon.es/10.3390/cells11192953.
Wu X, He W, Mu X, Liu Y, Deng J, Liu Y, et al. Macrophage polarization in diabetic wound healing. Burns Trauma. 2022;10:tkac051. https://doiorg.publicaciones.saludcastillayleon.es/10.1093/burnst/tkac051.
Weidenmaier C. Staphylococcus aureus blocks insulin function. Nat Microbiol. 2018;3(5):533–4. https://doiorg.publicaciones.saludcastillayleon.es/10.1038/s41564-018-0153-3.
Bäsler K, Bergmann S, Heisig M, Naegel A, Zorn-Kruppa M, Brandner JM. The role of tight junctions in skin barrier function and dermal absorption. J Control Release. 2016;242:105–18. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.jconrel.2016.08.007.
Kengmo Tchoupa A, Kretschmer D, Schittek B, Peschel A. The epidermal lipid barrier in microbiome-skin interaction. Trends Microbiol. 2023;31(7):723–34. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.tim.2023.01.009.
van den Bogaard EH, Elias PM, Goleva E, Berdyshev E, Smits JPH, Danby SG, et al. Targeting skin barrier function in atopic dermatitis. J Allergy Clin Immunol Pract. 2023;11(5):1335–46. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.jaip.2023.02.005.
Morizane S, Mukai T, Sunagawa K, Tachibana K, Kawakami Y, Ouchida M. Input/output cytokines in epidermal keratinocytes and the involvement in inflammatory skin diseases. Front Immunol. 2023;14:1239598. https://doiorg.publicaciones.saludcastillayleon.es/10.3389/fimmu.2023.1239598.
Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6). https://doiorg.publicaciones.saludcastillayleon.es/10.3390/ijms20061475.
Acknowledgements
I would like to express my appreciation to my co-authors and Yuqi Jiang, whose professional knowledge and experience were essential to the success of this paper. I would also like to extend my sincere thanks to the editors of the journal, whose efforts helped to refine and improve this study.
Funding
This research was supported by the National Natural Science Foundation of China [82202442], [82172199], [82401034], Shanghai Sailing Program [22YF1436700], the Fundamental Research Funds for the Central Universities [YG2025QNB18]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
DL, YSC, HX, and YL conceived and designed the study. DL, TYY, SM, ZS, LFS, WAW, YL, JAY and MG performed the experiments. WAW and SM analyzed the data. DL, TYY and SM drafted the manuscript. YSC, and YL provided critical revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethics Committee of Ruijin Hospital Shanghai Jiao Tong University School of Medicine (RJ2023021) and were conducted in compliance with relevant ethical regulations regarding animal testing and research. Patient consent statement: not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, D., Yu, T., Ma, S. et al. Insulin/PHMB-grafted sodium alginate hydrogels improve infected wound healing by antibacterial-prompted macrophage inflammatory regulation. J Nanobiotechnol 23, 328 (2025). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12951-025-03398-8
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12951-025-03398-8